Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy
- PMID: 36014304
- PMCID: PMC9414909
- DOI: 10.3390/molecules27165072
Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy
Abstract
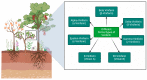
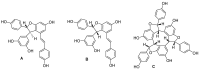
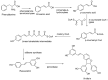
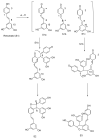
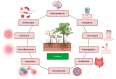
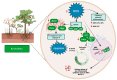
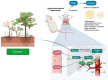
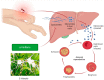
Viniferin is a resveratrol derivative. Resveratrol is the most prominent stilbenoid synthesized by plants as a defense mechanism in response to microbial attack, toxins, infections or UV radiation. Different forms of viniferin exist, including alpha-viniferin (α-viniferin), beta-viniferin (β-viniferin), delta-viniferin (δ-viniferin), epsilon-viniferin (ε-viniferin), gamma-viniferin (γ-viniferin), R-viniferin (vitisin A), and R2-viniferin (vitisin B). All of these forms exhibit a range of important biological activities and, therefore, have several possible applications in clinical research and future drug development. In this review, we present a comprehensive literature search on the chemistry and biosynthesis of and the diverse studies conducted on viniferin, especially with regards to its anti-inflammatory, antipsoriasis, antidiabetic, antiplasmodic, anticancer, anti-angiogenic, antioxidant, anti-melanogenic, neurodegenerative effects, antiviral, antimicrobial, antifungal, antidiarrhea, anti-obesity and anthelminthic activities. In addition to highlighting its important chemical and biological activities, coherent and environmentally acceptable methods for establishing vinferin on a large scale are highlighted to allow the development of further research that can help to exploit its properties and develop new phyto-pharmaceuticals. Overall, viniferin and its derivatives have the potential to be the most effective nutritional supplement and supplementary medication, especially as a therapeutic approach. More researchers will be aware of viniferin as a pharmaceutical drug as a consequence of this review, and they will be encouraged to investigate viniferin and its derivatives as pharmaceutical drugs to prevent future health catastrophes caused by a variety of serious illnesses.
Keywords: biosynthesis; chemistry; drug discovery; oligostilbenoid; pharmacology; viniferin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Plant-Derived Purification, Chemical Synthesis, and In Vitro/In Vivo Evaluation of a Resveratrol Dimer, Viniferin, as an HCV Replication Inhibitor.Viruses. 2019 Sep 23;11(10):890. doi: 10.3390/v11100890. Viruses. 2019. PMID: 31547617 Free PMC article.
-
In the shadow of resveratrol: biological activities of epsilon-viniferin.J Physiol Biochem. 2022 May;78(2):465-484. doi: 10.1007/s13105-022-00880-x. Epub 2022 Mar 21. J Physiol Biochem. 2022. PMID: 35312966 Review.
-
Resveratrol and its dimers ε-viniferin and δ-viniferin in red wine protect vascular endothelial cells by a similar mechanism with different potency and efficacy.Kaohsiung J Med Sci. 2020 Jul;36(7):535-542. doi: 10.1002/kjm2.12199. Epub 2020 Mar 2. Kaohsiung J Med Sci. 2020. PMID: 32118360 Free PMC article.
-
Percutaneous absorption of resveratrol and its oligomers to relieve psoriasiform lesions: In silico, in vitro and in vivo evaluations.Int J Pharm. 2020 Jul 30;585:119507. doi: 10.1016/j.ijpharm.2020.119507. Epub 2020 Jun 5. Int J Pharm. 2020. PMID: 32512223
-
Beneficial Effects of ε-Viniferin on Obesity and Related Health Alterations.Nutrients. 2023 Feb 12;15(4):928. doi: 10.3390/nu15040928. Nutrients. 2023. PMID: 36839286 Free PMC article. Review.
Cited by
-
Exosome-like Nanoparticles, High in Trans-δ-Viniferin Derivatives, Produced from Grape Cell Cultures: Preparation, Characterization, and Anticancer Properties.Biomedicines. 2024 Sep 20;12(9):2142. doi: 10.3390/biomedicines12092142. Biomedicines. 2024. PMID: 39335655 Free PMC article.
-
Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco-Vitis vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture.Molecules. 2023 May 13;28(10):4074. doi: 10.3390/molecules28104074. Molecules. 2023. PMID: 37241814 Free PMC article.
-
CRISPR/Cas9-driven double modification of grapevine MLO6-7 imparts powdery mildew resistance, while editing of NPR3 augments powdery and downy mildew tolerance.Plant J. 2025 Apr;122(2):e17204. doi: 10.1111/tpj.17204. Epub 2024 Dec 8. Plant J. 2025. PMID: 39645650 Free PMC article.
-
Phenolic Biotransformations in Wheatgrass Juice after Primary and Secondary Fermentation.Foods. 2023 Apr 12;12(8):1624. doi: 10.3390/foods12081624. Foods. 2023. PMID: 37107419 Free PMC article.
-
Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings by Freeze-Drying: Release Kinetics and In Vitro Bioaccessibility Assessment of Phenolic Compounds.Gels. 2024 May 21;10(6):353. doi: 10.3390/gels10060353. Gels. 2024. PMID: 38920899 Free PMC article.
References
-
- Ramawat K.G., Mérillon J.-M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Springer; Berlin/Heidelberg, Germany: 2013.
-
- Houillé B., Besseau S., Delanoue G., Oudin A., Papon N., Clastre M., Simkin A.J., Guerin L., Courdavault V., Giglioli-Guivarc’h N. Composition and tissue-specific distribution of stilbenoids in grape canes are affected by downy mildew pressure in the vineyard. J. Agric. Food Chem. 2015;63:8472–8477. doi: 10.1021/acs.jafc.5b02997. - DOI - PubMed
-
- González-Barrio R., Beltrán D., Cantos E., Gil M.I., Espín J.C., Tomás-Barberán F.A. Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. ‘Superior’ white table grapes. J. Agric. Food Chem. 2006;54:4222–4228. doi: 10.1021/jf060160f. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

