Maize Flavonoid Biosynthesis, Regulation, and Human Health Relevance: A Review
- PMID: 36014406
- PMCID: PMC9413827
- DOI: 10.3390/molecules27165166
Maize Flavonoid Biosynthesis, Regulation, and Human Health Relevance: A Review
Abstract
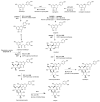
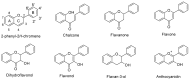
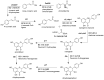
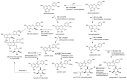
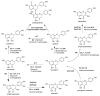
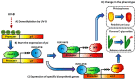
Maize is one of the most important crops for human and animal consumption and contains a chemical arsenal essential for survival: flavonoids. Moreover, flavonoids are well known for their beneficial effects on human health. In this review, we decided to organize the information about maize flavonoids into three sections. In the first section, we include updated information about the enzymatic pathway of maize flavonoids. We describe a total of twenty-one genes for the flavonoid pathway of maize. The first three genes participate in the general phenylpropanoid pathway. Four genes are common biosynthetic early genes for flavonoids, and fourteen are specific genes for the flavonoid subgroups, the anthocyanins, and flavone C-glycosides. The second section explains the tissue accumulation and regulation of flavonoids by environmental factors affecting the expression of the MYB-bHLH-WD40 (MBW) transcriptional complex. The study of transcription factors of the MBW complex is fundamental for understanding how the flavonoid profiles generate a palette of colors in the plant tissues. Finally, we also include an update of the biological activities of C3G, the major maize anthocyanin, including anticancer, antidiabetic, and antioxidant effects, among others. This review intends to disclose and integrate the existing knowledge regarding maize flavonoid pigmentation and its relevance in the human health sector.
Keywords: Zea mays L.; anthocyanins; biosynthesis; health benefits; pigmented maize; regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dewick P.M. Medicinal Natural Products: A Biosynthetic Approach. 3rd ed. John Wiley & Sons, Ltd.; Chichester, UK: 2009.
-
- Rauter A.P., Ennis M., Hellwich K.-H., Herold B.J., Horton D., Moss G.P., Schomburg I. Nomenclature of Flavonoids (IUPAC Recommendations 2017) Pure Appl. Chem. 2018;90:1429–1486. doi: 10.1515/pac-2013-0919. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

