Phytochemical Composition of Lichen Parmotrema hypoleucinum (J. Steiner) Hale from Algeria
- PMID: 36014465
- PMCID: PMC9416662
- DOI: 10.3390/molecules27165229
Phytochemical Composition of Lichen Parmotrema hypoleucinum (J. Steiner) Hale from Algeria
Abstract
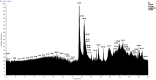
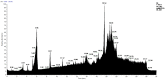
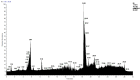
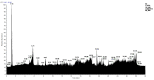
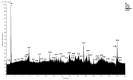
In this work, we carried out studies of the chemical composition of hexane, chloroform and ethanol extracts from two samples of the lichen Parmotrema hypoleucinum collected in Algeria. Each sample of the lichen P. hypoleucinum was collected on two different supports: Olea europaea and Quercus coccifera. Hexane extracts were prepared, in Soxhlet; each hexane extract was fractionated by its solubility in methanol; the products soluble in methanol were separated (cold): 1-Hexane, 2-Hexane; and the products insoluble in methanol (cold): 1-Cires, 2-Cires. A diazomethane esterified sample of 1-Hexane, 2-Hexane, 1-Cires and 2-Cires was analyzed by GC-MS, and the components were identified as methyl esters. In the 1-Hexane and 2-Hexane fractions, the methyl esters of the predominant fatty acids in the lichen were identified: palmitic acid, linoleic acid, oleic acid and stearic acid; a hydrocarbon was also identified: 13-methyl-17-norkaur-15-ene and several derivatives of orsellinic acid. In the 1-Cires and 2-Cires fractions, the previous fatty acids were no longer observed, and only the derivatives of orsellinic acid were found. The analysis of the 1-Hexane, 2-Hexane fractions by HPLC-MS/MS allows us to identify different chemical components, and the most characteristic products of the lichen were identified, such as Atranol, Chloroatranol, Atranorin and Chloroatranorin. In the fractions of 1-Cires and 2-Cires, the HPLC-MS/MS analysis reveals that they are very similar in their chemical components; the characteristic products of this lichen in this fraction are Atranorin and Chloroatranorin. In the extracts of chloroform, 1-Chloroform and 2-Chloroform, the analysis carried out by HPLC-MS/MS shows small differences in their chemical composition at the level of secondary products; among the products to be highlighted for this work, we have chloroatranorin, the stictic acid, norstictic acid and other derivatives. In the analysis of the most polar extracts carried out in ethanol: 1-Ethanol and 2-Ethanol, HPLC-MS/MS analysis shows very similar chemical compositions in these two extracts with small differences. In these extracts, the following acids were identified as characteristic compounds of this lichen: constictic acid, stictic acid, substictic acid and methylstictic acid. In the HPLC-MS/MS analysis of all these extracts, alectoronic acid was not found.
Keywords: LC-MSD-Trap-XCT; Parmotrema hypoleucinum; lichen; norstictic acid and stictic acid; phytochemical composition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Nash T.H. Lichen Biology. Cambridge University Press; Cambridge, UK: 2008. p. 498.
-
- Ranković B. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential. 2nd ed. Springer; Berlin/Heidelberg, Germany: 2019.
-
- Tehler A., Wedin M. Lichen Biology. 2nd ed. Cambridge University Press; London, UK: 2008. Systematics of Lichenized Fungi; pp. 336–352. Chapter 17.
-
- Nguyen K.V., Thi Do N.T., Chandna A., Pham C.V., Doan P.M., Nguyen A.Q., Nguyen C.K.T., Larsson M., Escalante S., Olowokure B., et al. Antibiotic use and resistance in emerging economies: A situation analysis for Viet Nam. BMC Public Health. 2013;13:1158. doi: 10.1186/1471-2458-13-1158. - DOI - PMC - PubMed
-
- Lücking R., Hodkinson B.P., Leavitt S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist. 2017;119:361–416. doi: 10.1639/0007-2745-119.4.361. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

