Structural and Functional Studies of S-(2-Carboxyethyl)-L-Cysteine and S-(2-Carboxyethyl)-l-Cysteine Sulfoxide
- PMID: 36014554
- PMCID: PMC9414067
- DOI: 10.3390/molecules27165317
Structural and Functional Studies of S-(2-Carboxyethyl)-L-Cysteine and S-(2-Carboxyethyl)-l-Cysteine Sulfoxide
Abstract
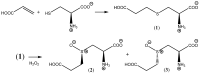
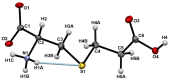
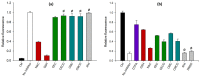
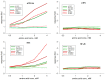
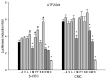
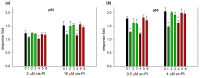
Insecticidal non-proteinogenic amino acid S-(2-carboxyethyl)-L-cysteine (β-CEC) and its assumed metabolite, S-(2-carboxyethyl)-l-cysteine sulfoxide (β-CECO), are present abundantly in a number of plants of the legume family. In humans, these amino acids may occur as a result of exposure to environmental acrylonitrile or acrylamide, and due to consumption of the legumes. The β-CEC molecule is a homolog of S-carboxymethyl-l-cysteine (carbocisteine, CMC), a clinically employed antioxidant and mucolytic drug. We report here detailed structural data for β-CEC and β-CECO, as well as results of in vitro studies evaluating cytotoxicity and the protective potential of the amino acids in renal tubular epithelial cells (RTECs) equipped with reporters for activity of seven stress-responsive transcription factors. In RTECs, β-CEC and the sulfoxide were not acutely cytotoxic, but activated the antioxidant Nrf2 pathway. β-CEC, but not the sulfoxide, induced the amino acid stress signaling, which could be moderated by cysteine, methionine, histidine, and tryptophan. β-CEC enhanced the cytotoxic effects of arsenic, cadmium, lead, and mercury, but inhibited the cytotoxic stress induced by cisplatin, oxaliplatin, and CuO nanoparticles and acted as an antioxidant in a copper-dependent oxidative DNA degradation assay. In these experiments, the structure and activities of β-CEC closely resembled those of CMC. Our data suggest that β-CEC may act as a mild activator of the cytoprotective pathways and as a protector from platinum drugs and environmental copper cytotoxicity.
Keywords: NRK-52E cell line; X-ray diffraction crystallography; amino acid stress signaling; antioxidants; carbocisteine; green fluorescent protein; heavy metal cytotoxicity; luciferase assay; transcriptional activation reporters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
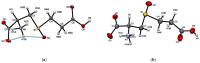


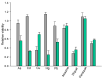
Similar articles
-
Structure, Antioxidant and Anti-inflammatory Activities of the (4R)- and (4S)-epimers of S-Carboxymethyl-L-cysteine Sulfoxide.Pharmaceuticals (Basel). 2020 Sep 25;13(10):270. doi: 10.3390/ph13100270. Pharmaceuticals (Basel). 2020. PMID: 32992738 Free PMC article.
-
Oxidative deamination of S-(1-carboxyethyl)-L-cysteine and S-(1-carboxypropyl)-L-cysteine by L-aminoacid oxidase.Ital J Biochem. 1986 Nov-Dec;35(6):385-90. Ital J Biochem. 1986. PMID: 3570717
-
An occupational hygiene investigation of exposure to acrylamide and the role for urinary S-carboxyethyl-cysteine (CEC) as a biological marker.Ann Occup Hyg. 2005 Nov;49(8):683-90. doi: 10.1093/annhyg/mei028. Epub 2005 Sep 1. Ann Occup Hyg. 2005. PMID: 16141254
-
S-carboxymethyl-L-cysteine.Drug Metab Rev. 2012 May;44(2):129-47. doi: 10.3109/03602532.2011.631015. Drug Metab Rev. 2012. PMID: 22497630 Review.
-
The deficiency of sulfoxidation of S-carboxymethyl-L-cysteine.Pharmacol Ther. 1989;43(2):237-49. doi: 10.1016/0163-7258(89)90120-4. Pharmacol Ther. 1989. PMID: 2675135 Review. No abstract available.
Cited by
-
(S)-2-Carb-oxy-ethyl l-cysteinyl sulfone.IUCrdata. 2024 May 31;9(Pt 5):x240480. doi: 10.1107/S2414314624004802. eCollection 2024 May. IUCrdata. 2024. PMID: 38846556 Free PMC article.
References
-
- Romeo J.T., Simmonds M.S.J. Nonprotein amino acid feeding deterrents from Calliandra. ACS Symp. Ser. 1989;387:59–68.
-
- Harwood C.E. Human food potential of the seeds of some Australian dry–zone Acacia species. J. Arid Environ. 1994;27:27–35. doi: 10.1006/jare.1994.1042. - DOI
-
- Seneviratne A.S., Fowden L. The amino acids of the genus Acacia. Phytochemistry. 1968;7:1039–1045. doi: 10.1016/S0031-9422(00)88249-7. - DOI
-
- Bull P.J., Brooke R.K., Cocker J., Jones K., Warren N. An occupational hygiene investigation of exposure to acrylamide and the role for urinary S-carboxyethyl-cysteine (CEC) as a biological marker. Ann. Occup. Hyg. 2005;49:683–690. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

