Photocatalytic Isomerization of (E)-Anethole to (Z)-Anethole
- PMID: 36014580
- PMCID: PMC9412280
- DOI: 10.3390/molecules27165342
Photocatalytic Isomerization of (E)-Anethole to (Z)-Anethole
Abstract
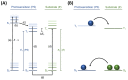
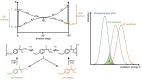
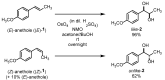
Natural product (E)-anethole was isomerized to (Z)-anethole in a photocatalytic reaction. For this purpose, a self-designed cheap photoreactor was constructed. Among 11 photosensitizers (organo and metal complex compounds), Ir(p-tBu-ppy)3 led to the highest conversion. Triplet energies of (E)- and (Z)-anethole were predicted theoretically by DFT calculations to support the selection of appropriate photosensitizers. A catalyst loading of 0.1 mol% gave up to 90% conversion in gram scale. Further additives were not required and mild irradiation with light of 400 nm overnight was sufficient. As a proof of concept, (E)- and (Z)-anethole were dihydroxylated diastereoselectively to obtain diastereomerically pure like- and unlike-configured diols, respectively.
Keywords: (E/Z)-isomerization; DFT calculations; calculation of triplet energy; catalysis; diastereoselective dihydroxylation; green chemistry; natural product; photocatalysis; photosensitizer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Duță D.E., Culețu A., Negoiță M., Ionescu V. Quantification of Anethole in Fennel and Anise Essential Oils using Gas Chromatography and 1H-NMR-Spectroscopy. Bull. UASVM Food Sci. Technol. 2019;76:105–113. doi: 10.15835/buasvmcn-fst:2019.0020. - DOI
-
- Angle S.R., Arnaiz D.O. Formal [3 + 2]cycloaddition of benzylic cations with alkenes. J. Org. Chem. 1992;57:5937–5947. doi: 10.1021/jo00048a029. - DOI
-
- Goez M., Eckert G. Photoinduced Electron Transfer Reactions of Aryl Olefins. Cis-Trans Isomerization and Cycloadduct Formation in Anethole-Fumaronitrile Systems in Polar Solvents. J. Am. Chem. Soc. 1996;118:140–154. doi: 10.1021/ja9511578. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

