Studies of the Structure and Optical Properties of BaSrMgWO6 Thin Films Deposited by a Spin-Coating Method
- PMID: 36014622
- PMCID: PMC9414463
- DOI: 10.3390/nano12162756
Studies of the Structure and Optical Properties of BaSrMgWO6 Thin Films Deposited by a Spin-Coating Method
Abstract
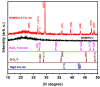
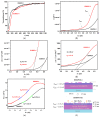
Highly transparent thin films with the chemical formula BaSrMgWO6 were deposited by spin coating using a solution of nitrates of Ba, Sr, and Mg and ammonium paratungstate in dimethylformamide with a Ba:Sr:Mg:W ratio = 1:1:1:1. XRD, SEM, EDX, and XPS investigations evidenced that annealing at 800 °C for 1 h results in an amorphous structure having a precipitate on its surface, and that supplementary annealing at 850 °C for 45 min forms a nanocrystalline structure and dissolves a portion of the precipitates. A textured double perovskite cubic structure (61.9%) was found, decorated with tetragonal and cubic impurity phases (12.7%), such as BaO2, SrO2, and MgO, and an under-stoichiometric phase (24.4%) with the chemical formula Ba2-(x+y) SrxMgyWO5. From transmittance measurements, the values of the optical band gap were estimated for the amorphous (Egdir = 5.21 eV, Egind = 3.85 eV) and nanocrystalline (Egdir = 4.69 eV, Egind = 3.77 eV) phases. The presence of a lattice disorder was indicated by the high Urbach energy values and weak absorption tail energies. A decrease in their values was observed and attributed to the crystallization process, lattice strain diminution, and cation redistribution.
Keywords: double perovskite; optical properties; structure; thin films; tungstate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Galasso F.S. Structure, Properties and Preparation of Perovskite-Type Compounds. Pergamon Press; Oxford, UK: 1969.
-
- Doroftei C., Popa P.D., Iacomi F., Leontie L. The influence of Zn2+ ions on the microstructure, electrical and gas sensing properties of La0.8Pb0.2FeO3 perovskite. Sens. Actuators B Chem. 2014;191:239–245. doi: 10.1016/j.snb.2013.09.113. - DOI
-
- Rezlescu N., Rezlescu E., Popa P.D., Doroftei C., Ignat M. Partial substitution of manganese with cerium in SrMnO3 nano-perovskite catalyst. Effect of the modification on the catalytic combustion of dilute acetone. Mater. Chem. Phys. 2016;182:332–337. doi: 10.1016/j.matchemphys.2016.07.040. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

