Neuroprotective Natural Products' Regulatory Effects on Depression via Gut-Brain Axis Targeting Tryptophan
- PMID: 36014776
- PMCID: PMC9413544
- DOI: 10.3390/nu14163270
Neuroprotective Natural Products' Regulatory Effects on Depression via Gut-Brain Axis Targeting Tryptophan
Abstract
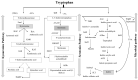
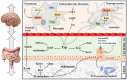
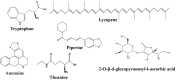
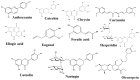
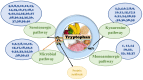
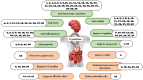
L-tryptophan (Trp) contributes to regulating bilateral communication of the gut-brain axis. It undergoes three major metabolic pathways, which lead to formation of kynurenine, serotonin (5-HT), and indole derivatives (under the control of the microbiota). Metabolites from the principal Trp pathway, kynurenic acid and quinolinic acid, exhibit neuroprotective activity, while picolinic acid exhibits antioxidant activity, and 5-HT modulates appetite, sleep cycle, and pain. Abnormality in Trp plays crucial roles in diseases, including depression, colitis, ulcer, and gut microbiota-related dysfunctions. To address these diseases, the use of natural products could be a favorable alternative because they are a rich source of compounds that can modulate the activity of Trp and combat various diseases through modulating different signaling pathways, including the gut microbiota, kynurenine pathway, and serotonin pathway. Alterations in the signaling cascade pathways via different phytochemicals may help us explore the deep relationships of the gut-brain axis to study neuroprotection. This review highlights the roles of natural products and their metabolites targeting Trp in different diseases. Additionally, the role of Trp metabolites in the regulation of neuroprotective and gastroprotective activities is discussed. This study compiles the literature on novel, potent neuroprotective agents and their action mechanisms in the gut-brain axis and proposes prospective future studies to identify more pharmaceuticals based on signaling pathways targeting Trp.
Keywords: 5-HT; L-tryptophan; gastroprotective; gut–brain axis; metabolites; neuroprotective; phytochemicals; signaling pathways.
Conflict of interest statement
The authors declared that there is no potential conflict of interest.
Figures
Similar articles
-
Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain.Gut Microbes. 2021 Jan-Dec;13(1):1-16. doi: 10.1080/19490976.2020.1869501. Gut Microbes. 2021. PMID: 33535879 Free PMC article.
-
A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression.Stress. 2008 May;11(3):198-209. doi: 10.1080/10253890701754068. Stress. 2008. PMID: 18465467 Review.
-
Role of Probiotics in Depression: Connecting Dots of Gut-Brain-Axis Through Hypothalamic-Pituitary Adrenal Axis and Tryptophan/Kynurenic Pathway involving Indoleamine-2,3-dioxygenase.Mol Neurobiol. 2025 Jun;62(6):7230-7241. doi: 10.1007/s12035-025-04708-9. Epub 2025 Jan 28. Mol Neurobiol. 2025. PMID: 39875781 Review.
-
Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice.Microbiome. 2023 Jun 30;11(1):145. doi: 10.1186/s40168-023-01589-9. Microbiome. 2023. PMID: 37386523 Free PMC article.
-
Tryptophan-kynurenine metabolic characterization in the gut and brain of depressive-like rats induced by chronic restraint stress.J Affect Disord. 2023 May 1;328:273-286. doi: 10.1016/j.jad.2023.02.008. Epub 2023 Feb 4. J Affect Disord. 2023. PMID: 36746244
Cited by
-
Recent Progress in Mass Spectrometry-Based Metabolomics in Major Depressive Disorder Research.Molecules. 2023 Nov 4;28(21):7430. doi: 10.3390/molecules28217430. Molecules. 2023. PMID: 37959849 Free PMC article. Review.
-
The Role of Mitochondrial Dysfunction-Mediated Changes in Immune Cytokine Expression in the Pathophysiology and Treatment of Major Depressive Disorder.Mol Neurobiol. 2025 Aug;62(8):9817-9828. doi: 10.1007/s12035-025-04872-y. Epub 2025 Mar 31. Mol Neurobiol. 2025. PMID: 40163267 Review.
-
A systematic review on the use of phytotherapy in managing clinical depression.Bioimpacts. 2024 Aug 11;15:30532. doi: 10.34172/bi.30532. eCollection 2025. Bioimpacts. 2024. PMID: 40256221 Free PMC article. Review.
-
Exploring the interplay between running exercises, microbial diversity, and tryptophan metabolism along the microbiota-gut-brain axis.Front Microbiol. 2024 Jan 22;15:1326584. doi: 10.3389/fmicb.2024.1326584. eCollection 2024. Front Microbiol. 2024. PMID: 38318337 Free PMC article.
-
A review of the pharmacological action and mechanism of natural plant polysaccharides in depression.Front Pharmacol. 2024 Feb 8;15:1348019. doi: 10.3389/fphar.2024.1348019. eCollection 2024. Front Pharmacol. 2024. PMID: 38389919 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

