Serum Vitamin D Levels Explored in the Latvian Cohort of Patients with Basal Cell Carcinoma Linked to the Sonic Hedgehog and Vitamin D Binding Protein Cutaneous Tissue Indices
- PMID: 36014865
- PMCID: PMC9413259
- DOI: 10.3390/nu14163359
Serum Vitamin D Levels Explored in the Latvian Cohort of Patients with Basal Cell Carcinoma Linked to the Sonic Hedgehog and Vitamin D Binding Protein Cutaneous Tissue Indices
Abstract
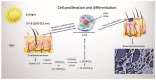
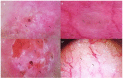
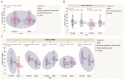
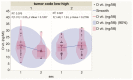
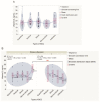
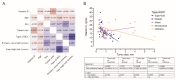
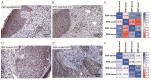
Ultraviolet radiation is known as one of the major contributors to skin malignancies, including basal cell carcinoma (BCC), which is the most common type of skin cancer. It is a heterogeneous tumor, which presents with various types that are stratified into low- and high-risk tumors. Sunlight is important for overall health and vitamin D synthesis in the skin, whereas deviations from the optimal level of vitamin D are shown to be associated with the risk of the development of BCC. The accumulating evidence suggests the ability of vitamin D to antagonize the Sonic Hedgehog (SHH) signaling, the key tumor pathway, and play a protective role in the development of BCC. Additionally, a vitamin D binding protein (DBP) is shown to be implicated in the complex regulation of vitamin D. Here, we aimed to explore serum vitamin D in patients with different primary and recurrent BCC of the head and neck and investigate cutaneous DBP and SHH indices, confirmed immunohistochemically in these subjects. According to the results, 94.9% of the Latvian cohort of BCC patients were found to be deficient in vitamin D. No significant differences in serum vitamin D levels were found between genders, primary and recurrent tumors, and different types of BCC. Serum vitamin D was inversely associated with tumor size. Susceptible male individuals with low blood vitamin D levels were recognized at risk of developing aggressive and recurrent BCC confirmed by the use of hierarchical clustering analysis. In smaller tumors with a favorable course, such as superficial and nodular BCC, the association between high DBP and low SHH tissue expression was found, providing supportive evidence of the existence of a link between vitamin D, proteins involved in its metabolism, as exemplified by the DBP and SHH signaling pathway. The assumption of a deficiency in the protective effect of vitamin D in patients with high-risk BCCs was proposed in low DBP and high SHH tissue indices. New extensions to existing knowledge and characterization of the BCC signaling pathways and their cross-talk with vitamin D are warranted when searching for a preferential effect of vitamin D on skin cancer.
Keywords: Sonic Hedgehog; basal cell carcinoma; hierarchical clustering; immunohistochemistry; serum levels of vitamin D; ultraviolet radiation; vitamin D binding protein; vitamin D deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Situm M., Buljan M., Bulat V., Lugović-Mihić L., Bolanca Z., Simić D. The role of UV radiation in the development of basal cell carcinoma. Coll. Antropol. 2008;32:167–170. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

