The Modulation of Ubiquinone, a Lipid Antioxidant, on Neuronal Voltage-Gated Sodium Current
- PMID: 36014898
- PMCID: PMC9413396
- DOI: 10.3390/nu14163393
The Modulation of Ubiquinone, a Lipid Antioxidant, on Neuronal Voltage-Gated Sodium Current
Abstract
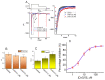
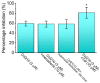
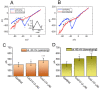
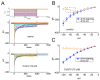
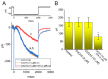
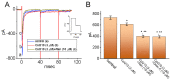
Ubiquinone, composed of a 1,4-benzoquinone and naturally produced in the body, actively participates in the mitochondrial redox reaction and functions as an endogenous lipid antioxidant, protecting against peroxidation in the pituitary-dependent hormonal system. However, the questions of if and how ubiquinone directly affects neuronal ionic currents remain largely unsettled. We investigated its effects on ionic currents in pituitary neurons (GH3 and MMQ cells) with the aid of patch-clamp technology. Ubiquinone decreased the peak amplitude of the voltage-gated Na+ current (INa) with a slowing of the inactivation rate. Neither menadione nor superoxide dismutase modified the ubiquinone-induced INa inhibition. In response to an isosceles-triangular ramp pulse, the persistent INa (INa(P)) at high- and low- threshold potentials occurred concurrently with a figure-eight hysteresis loop. With ubiquinone, the INa(P) increased with no change in the intersection voltage, and the magnitude of the voltage-dependent hysteresis of the current was enhanced. Ubiquinone was ineffective in modifying the gating of hyperpolarization-activated cation currents. In MMQ lactotrophs, ubiquinone effectively decreased the amplitude of the INa and the current inactivation rate. In sum, the effects of ubiquinone demonstrated herein occur upstream of its effects on mitochondrial redox processes, involved in its modulation of sodium channels and neuronal excitability.
Keywords: coenzyme Q10; current kinetics; persistent Na+ current; ubiquinone; voltage hysteresis; voltage-gated Na+ current.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

