SARS-CoV-2 and Emerging Foodborne Pathogens: Intriguing Commonalities and Obvious Differences
- PMID: 36014958
- PMCID: PMC9415055
- DOI: 10.3390/pathogens11080837
SARS-CoV-2 and Emerging Foodborne Pathogens: Intriguing Commonalities and Obvious Differences
Abstract
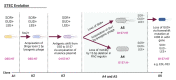
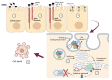
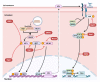
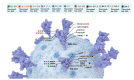
The coronavirus disease 2019 (COVID-19) has resulted in tremendous human and economic losses around the globe. The pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a virus that is closely related to SARS-CoV and other human and animal coronaviruses. Although foodborne diseases are rarely of pandemic proportions, some of the causative agents emerge in a manner remarkably similar to what was observed recently with SARS-CoV-2. For example, Shiga toxin-producing Escherichia coli (STEC), the most common cause of hemolytic uremic syndrome, shares evolution, pathogenesis, and immune evasion similarities with SARS-CoV-2. Both agents evolved over time in animal hosts, and during infection, they bind to specific receptors on the host cell's membrane and develop host adaptation mechanisms. Mechanisms such as point mutations and gene loss/genetic acquisition are the main driving forces for the evolution of SARS-CoV-2 and STEC. Both pathogens affect multiple body organs, and the resulting diseases are not completely cured with non-vaccine therapeutics. However, SARS-CoV-2 and STEC obviously differ in the nature of the infectious agent (i.e., virus vs. bacterium), disease epidemiological details (e.g., transmission vehicle and symptoms onset time), and disease severity. SARS-CoV-2 triggered a global pandemic while STEC led to limited, but sometimes serious, disease outbreaks. The current review compares several key aspects of these two pathogenic agents, including the underlying mechanisms of emergence, the driving forces for evolution, pathogenic mechanisms, and the host immune responses. We ask what can be learned from the emergence of both infectious agents in order to alleviate future outbreaks or pandemics.
Keywords: COVID-19; SARS-CoV-2; Shiga toxin; Shiga toxin-producing Escherichia coli; infectious diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Comparative Genomics of Shiga Toxin-Producing Escherichia coli Strains Isolated from Pediatric Patients with and without Hemolytic Uremic Syndrome from 2000 to 2016 in Finland.Microbiol Spectr. 2022 Aug 31;10(4):e0066022. doi: 10.1128/spectrum.00660-22. Epub 2022 Jun 22. Microbiol Spectr. 2022. PMID: 35730965 Free PMC article.
-
Role of Recent Therapeutic Applications and the Infection Strategies of Shiga Toxin-Producing Escherichia coli.Front Cell Infect Microbiol. 2021 Jun 29;11:614963. doi: 10.3389/fcimb.2021.614963. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34268129 Free PMC article. Review.
-
Investigations of Possible Multistate Outbreaks of Salmonella, Shiga Toxin-Producing Escherichia coli, and Listeria monocytogenes Infections - United States, 2016.MMWR Surveill Summ. 2020 Nov 13;69(6):1-14. doi: 10.15585/mmwr.ss6906a1. MMWR Surveill Summ. 2020. PMID: 33180756 Free PMC article.
-
Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity.Cells. 2021 Oct 29;10(11):2949. doi: 10.3390/cells10112949. Cells. 2021. PMID: 34831172 Free PMC article. Review.
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
References
-
- National Institute of Health (NIH) Clinical Spectrum of SARS-CoV-2 Infection. [(accessed on 31 March 2022)];2021 Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
-
- World Health Organization . WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. World Health Organization; Geneva, Switzerland: 2015. [(accessed on 24 March 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/199350/?sequence=1.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

