Interferon Epsilon Signaling Confers Attenuated Zika Replication in Human Vaginal Epithelial Cells
- PMID: 36014974
- PMCID: PMC9415962
- DOI: 10.3390/pathogens11080853
Interferon Epsilon Signaling Confers Attenuated Zika Replication in Human Vaginal Epithelial Cells
Abstract
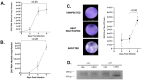
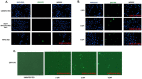
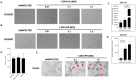
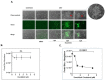
Zika virus (ZIKV) is an emerging flavivirus that causes congenital birth defects and neurological compilations in the human host. Although ZIKV is primarily transmitted through infected mosquitos, recent studies reveal sexual contact as a potential transmission route. In vagina-bearing individuals, the vaginal epithelium constitutes the first line of defense against viruses. However, it is unclear how ZIKV interacts with the vaginal epithelium to initiate ZIKV transmission. In this study, we demonstrate that exposing ZIKV to human vaginal epithelial cells (hVECs) resulted in de novo viral RNA replication, increased envelope viral protein production, and a steady, extracellular release of infectious viral particles. Interestingly, our data show that, despite an increase in viral load, the hVECs did not exhibit significant cytopathology in culture as other cell types typically do. Furthermore, our data reveal that the innate antiviral state of hVECs plays a crucial role in preventing viral cytopathology. For the first time, our data show that interferon epsilon inhibits ZIKV replication. Collectively, our results in this study provide a novel perspective on the viral susceptibility and replication dynamics during ZIKV infection in the human vaginal epithelium. These findings will be instrumental towards developing therapeutic agents aimed at eliminating the pathology caused by the virus.
Keywords: Zika virus; human vaginal epithelial cells; interferon epsilon; primary cervical cell; sexual transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Zika Virus Replicates in the Vagina of Mice with Intact Interferon Signaling.J Virol. 2022 Sep 28;96(18):e0121922. doi: 10.1128/jvi.01219-22. Epub 2022 Aug 30. J Virol. 2022. PMID: 36040178 Free PMC article.
-
Maternal Zika Virus (ZIKV) Infection following Vaginal Inoculation with ZIKV-Infected Semen in Timed-Pregnant Olive Baboons.J Virol. 2020 May 18;94(11):e00058-20. doi: 10.1128/JVI.00058-20. Print 2020 May 18. J Virol. 2020. PMID: 32188737 Free PMC article.
-
Zika Virus Subgenomic Flavivirus RNA Generation Requires Cooperativity between Duplicated RNA Structures That Are Essential for Productive Infection in Human Cells.J Virol. 2020 Aug 31;94(18):e00343-20. doi: 10.1128/JVI.00343-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32581095 Free PMC article.
-
Hide and Seek: The Interplay Between Zika Virus and the Host Immune Response.Front Immunol. 2021 Oct 21;12:750365. doi: 10.3389/fimmu.2021.750365. eCollection 2021. Front Immunol. 2021. PMID: 34745123 Free PMC article. Review.
-
Zika virus and reproduction: facts, questions and current management.Hum Reprod Update. 2017 Nov 1;23(6):629-645. doi: 10.1093/humupd/dmx024. Hum Reprod Update. 2017. PMID: 28961800 Review.
Cited by
-
Superior antiviral activity of IFNβ in genital HSV-1 infection.Front Cell Infect Microbiol. 2022 Oct 17;12:949036. doi: 10.3389/fcimb.2022.949036. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36325470 Free PMC article.
-
Interferon Epsilon: Properties and Functions.Curr Mol Med. 2025;25(6):723-733. doi: 10.2174/0115665240309075240603062703. Curr Mol Med. 2025. PMID: 38859786 Review.
-
Trichomonas vaginalis extracellular vesicles suppress IFNε-mediated responses driven by its intracellular bacterial symbiont Mycoplasma hominis.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2508297122. doi: 10.1073/pnas.2508297122. Epub 2025 Jun 25. Proc Natl Acad Sci U S A. 2025. PMID: 40560611 Free PMC article.
-
Interferon Epsilon-Mediated Antiviral Activity Against Human Metapneumovirus and Respiratory Syncytial Virus.Vaccines (Basel). 2024 Oct 21;12(10):1198. doi: 10.3390/vaccines12101198. Vaccines (Basel). 2024. PMID: 39460364 Free PMC article.
-
Detection and persistence of Zika virus in body fluids and associated factors: a prospective cohort study.Sci Rep. 2023 Dec 6;13(1):21557. doi: 10.1038/s41598-023-48493-8. Sci Rep. 2023. PMID: 38057382 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

