Pharmacokinetic Interaction between Atorvastatin and Omega-3 Fatty Acid in Healthy Volunteers
- PMID: 36015110
- PMCID: PMC9415283
- DOI: 10.3390/ph15080962
Pharmacokinetic Interaction between Atorvastatin and Omega-3 Fatty Acid in Healthy Volunteers
Abstract
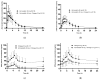
The interaction between statins and omega-3 fatty acids remains controversial. The aim of this phase 1 trial was to evaluate the pharmacokinetics of drug-drug interaction between atorvastatin and omega-3 fatty acids. Treatments were once-daily oral administrations of omega-3 (4 g), atorvastatin (40 mg), and both for 14 days, 7 days, and 14 days, respectively, with washout periods. The concentrations of atorvastatin, 2-OH-atorvastatin, docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) were determined with LC-MS/MS. Parameters of DHA and EPA were analyzed after baseline correction. A total of 37 subjects completed the study without any major violations. The geometric mean ratios (GMRs) and 90% confidence intervals (CIs) of the co-administration of a single drug for the area under the concentration-time curve during the dosing interval at steady state of atorvastatin, 2-OH-atorvastatin, DHA, and EPA were 1.042 (0.971-1.118), 1.185 (1.113-1.262), 0.157 (0.091-0.271), and 0.557 (0.396-0.784), respectively. The GMRs (90% Cis) for the co-administration at steady state of atorvastatin, 2-OH-atorvastatin, DHA, and EPA were 1.150 (0.990-1.335), 1.301 (1.2707-1.1401), 0.320 (0.243-0.422), and 0.589 (0.487-0.712), respectively. The 90% CIs for most primary endpoints were outside the range of typical bioequivalence, indicating a pharmacokinetic interaction between atorvastatin and omega-3.
Keywords: atorvastatin; docosahexaenoic acid; drug-drug interaction; dyslipidemia; eicosapentaenoic acid; pharmacokinetics.
Conflict of interest statement
W.T.J., K-Y.N. and YS.K. are employees of Korea United Pharm., Inc. H.J.L. and JH.M. are employees of Caleb Multilab., Inc. Other authors declare no conflicts of interest.
Figures
Similar articles
-
Pharmacokinetic Comparison Between a Fixed-Dose Combination of Atorvastatin/Omega-3-Acid Ethyl Esters and the Corresponding Loose Combination in Healthy Korean Male Subjects.Drug Des Devel Ther. 2024 Feb 8;18:395-406. doi: 10.2147/DDDT.S435885. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38352172 Free PMC article. Clinical Trial.
-
Pharmacokinetics of Single and Multiple Doses of Omega-3 Carboxylic Acids in Healthy Chinese Subjects: A Phase I, Open-Label Study.Clin Pharmacol Drug Dev. 2020 Nov;9(8):985-994. doi: 10.1002/cpdd.800. Epub 2020 Jun 21. Clin Pharmacol Drug Dev. 2020. PMID: 32567203 Clinical Trial.
-
Bioequivalence of two omega-3 fatty acid ethyl ester formulations: a case of clinical pharmacology of dietary supplements.Br J Clin Pharmacol. 2012 Jul;74(1):60-5. doi: 10.1111/j.1365-2125.2012.04174.x. Br J Clin Pharmacol. 2012. PMID: 22242645 Free PMC article. Clinical Trial.
-
Risk stratification by the "EPA+DHA level" and the "EPA/AA ratio" focus on anti-inflammatory and antiarrhythmogenic effects of long-chain omega-3 fatty acids.Herz. 2004 Nov;29(7):673-85. doi: 10.1007/s00059-004-2602-4. Herz. 2004. PMID: 15580322 Review.
-
Eicosapentaenoic Acid Versus Docosahexaenoic Acid as Options for Vascular Risk Prevention: A Fish Story.Am J Ther. 2016 May-Jun;23(3):e905-10. doi: 10.1097/MJT.0000000000000165. Am J Ther. 2016. PMID: 25828517 Review.
Cited by
-
Evaluating the effect of atorvastatin exposure and vitamin D levels on lipid outcomes in people with HIV-1 with suppressed HIV-1 RNA and LDL cholesterol <130 mg/dL.HIV Med. 2023 Jun;24(6):749-753. doi: 10.1111/hiv.13453. Epub 2022 Dec 22. HIV Med. 2023. PMID: 36549898 Free PMC article. Clinical Trial.
-
Pharmacokinetic Comparison Between a Fixed-Dose Combination of Atorvastatin/Omega-3-Acid Ethyl Esters and the Corresponding Loose Combination in Healthy Korean Male Subjects.Drug Des Devel Ther. 2024 Feb 8;18:395-406. doi: 10.2147/DDDT.S435885. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38352172 Free PMC article. Clinical Trial.
-
A Randomized, Open-Label, Single-Dose, Crossover Study of the Comparative Bioavailability of EPA and DHA in a Novel Liquid Crystalline Nanoparticle-Based Formulation of ω-3 Acid Ethyl Ester Versus Omacor® Soft Capsule among Healthy Adults.Int J Mol Sci. 2023 Dec 6;24(24):17201. doi: 10.3390/ijms242417201. Int J Mol Sci. 2023. PMID: 38139029 Free PMC article. Clinical Trial.
References
-
- Nissen S.E., Nicholls S.J., Sipahi I., Libby P., Raichlen J.S., Ballantyne C.M., Davignon J., Erbel R., Fruchart J.C., Tardif J.C., et al. Effect Of very high-intensity statin therapy on regression of coronary atherosclerosis—The ASTEROID trial. JAMA-J. Am. Med. Assoc. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. - DOI - PubMed
-
- Pedersen T.R., Kjekshus J., Berg K., Haghfelt T., Faergeman O., Thorgeirsson G., Pyorala K., Miettinen T., Wilhelmsen L., Olsson A.G., et al. Randomized trial of cholesterol-lowering in 4444 patients with coronary-heart-disease—The Scandinavian Simvastatin Survival Study (4s) Lancet. 1994;344:1383–1389. - PubMed
-
- Sacks F.M., Alaupovic P., Moye L.A., Cole T.G., Sussex B., Stampfer M.J., Pfeffer M.A., Braunwald E. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102:1886–1892. doi: 10.1161/01.CIR.102.16.1886. - DOI - PubMed
-
- Tonkin A.M., Glaziou P.P. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) study. Int. Congr. Ser. 1998;1155:231–237.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

