Impact of Sex on Proper Use of Inhaler Devices in Asthma and COPD: A Systematic Review and Meta-Analysis
- PMID: 36015191
- PMCID: PMC9414749
- DOI: 10.3390/pharmaceutics14081565
Impact of Sex on Proper Use of Inhaler Devices in Asthma and COPD: A Systematic Review and Meta-Analysis
Abstract
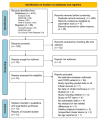
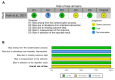
Despite females being more often affected by asthma than males and the prevalence of COPD rising in females, conflicting evidence exists as to whether sex may modulate the correct inhaler technique. The aim of this study was to assess the impact of sex on the proper use of inhaler devices in asthma and COPD. A pairwise meta-analysis was performed on studies enrolling adult males and females with asthma or COPD and reporting data of patients making at least one error by inhaler device type (DPI, MDI, and SMI). The data of 6,571 patients with asthma or COPD were extracted from 12 studies. A moderate quality of evidence (GRADE +++) indicated that sex may influence the correct use of inhaler device in both asthma and COPD. The critical error rate was higher in females with asthma (OR 1.31, 95%CI 1.14−1.50) and COPD (OR 1.80, 95%CI 1.22−2.67) using DPI vs. males (p < 0.01). In addition, the use of SMI in COPD was associated with a greater rate of critical errors in females vs. males (OR 5.36, 95%CI 1.48−19.32; p < 0.05). No significant difference resulted for MDI. In conclusion, choosing the right inhaler device in agreement with sex may optimize the pharmacological treatment of asthma and COPD.
Keywords: COPD; asthma; inhaler device; inhaler technique; meta-analysis; sex.
Conflict of interest statement
L.C. has participated as advisor in scientific meetings under the sponsorship of Boehringer Ingelheim and Novartis; received nonfinancial support from AstraZeneca; a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, No-vartis, and Almirall; is or has been a consultant to ABC Farmaceutici, Edmond Phar-ma, Zambon, Verona Pharma, and Ockham Biotech; and his department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, and Zambon. M.A. has no conflicts of interest to declare. A.F. has no conflicts of interest to declare. B.L.R. has no conflicts of interest to declare. E.P. has no conflicts of interest to declare. P.R. reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, grants and personal fees from Chiesi Farmaceutici, grants and personal fees from Almirall, grants from Zambon, personal fees from Biofutura, personal fees from GlaxoSmithKline, personal fees from Menarini, personal fees from Mundipharma. A.C. received grants from Menarini and AstraZeneca, and personal fees from Chiesi Farmaceutici.
Figures


References
-
- GINA 2021 GINA Main Report|Global Initiative for Asthma. [(accessed on 22 July 2021)]. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V....
-
- Asthma. [(accessed on 22 February 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma.
-
- GOLD Global Strategy for Prevention, Diagnosis and Management of COPD: 2022 Report. 2022. [(accessed on 14 March 2022)]. Available online: https://goldcopd.org/2022-gold-reports-2/
-
- Roche N., Plaza V., Backer V., van der Palen J., Cerveri I., Gonzalez C., Safioti G., Scheepstra I., Patino O., Singh D. Asthma Control and COPD Symptom Burden in Patients Using Fixed-Dose Combination Inhalers (SPRINT Study) NPJ Prim. Care Respir. Med. 2020;30:1. doi: 10.1038/s41533-019-0159-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

