Targeting Myocardial Fibrosis-A Magic Pill in Cardiovascular Medicine?
- PMID: 36015225
- PMCID: PMC9414721
- DOI: 10.3390/pharmaceutics14081599
Targeting Myocardial Fibrosis-A Magic Pill in Cardiovascular Medicine?
Abstract
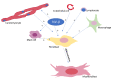
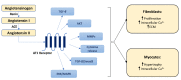
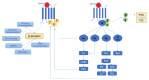
Fibrosis, characterized by an excessive accumulation of extracellular matrix, has long been seen as an adaptive process that contributes to tissue healing and regeneration. More recently, however, cardiac fibrosis has been shown to be a central element in many cardiovascular diseases (CVDs), contributing to the alteration of cardiac electrical and mechanical functions in a wide range of clinical settings. This paper aims to provide a comprehensive review of cardiac fibrosis, with a focus on the main pathophysiological pathways involved in its onset and progression, its role in various cardiovascular conditions, and on the potential of currently available and emerging therapeutic strategies to counteract the development and/or progression of fibrosis in CVDs. We also emphasize a number of questions that remain to be answered, and we identify hotspots for future research.
Keywords: antifibrotic strategies; cardiac fibrosis; cardiovascular diseases; fibrosis pathways; therapeutic strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

