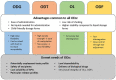
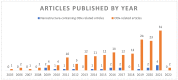
Orally Dispersible Dosage Forms for Paediatric Use: Current Knowledge and Development of Nanostructure-Based Formulations
- PMID: 36015247
- PMCID: PMC9414456
- DOI: 10.3390/pharmaceutics14081621
Orally Dispersible Dosage Forms for Paediatric Use: Current Knowledge and Development of Nanostructure-Based Formulations
Abstract
The paediatric population has always suffered from a lack of medicines tailored to their needs, especially in terms of accurate dosage, stability and acceptability. Orodispersible dosage forms have gone through a resurrection as an alternative to liquid formulations or fractioned solid formulations, although they are still subject to several inconveniences, among which the unpleasant taste and the low oral bioavailability of the API are the most significant hurdles in the way of achieving an optimal drug product. Nanostructures can address these inconveniences through their size and variety, owing to the plethora of materials that can be used in their manufacturing. Through the formation and functionalisation of nanostructures, followed by their inclusion in orodispersible dosage forms, safe, stable and acceptable medicines intended for paediatric use can be developed.
Keywords: nanostructure; oral bioavailability; orodispersible dosage forms; paediatric medicines; polymeric nanoparticles; taste-masking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- European Commission Regulation (EC) No. 1901/2006 of the European Parliament and of the Council of 12 December 2006 on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No. 726/2004. [(accessed on 10 February 2022)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006R1901&q....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources