Ruthenium(II) Polypyridyl Complexes for Antimicrobial Photodynamic Therapy: Prospects for Application in Cystic Fibrosis Lung Airways
- PMID: 36015290
- PMCID: PMC9412327
- DOI: 10.3390/pharmaceutics14081664
Ruthenium(II) Polypyridyl Complexes for Antimicrobial Photodynamic Therapy: Prospects for Application in Cystic Fibrosis Lung Airways
Abstract
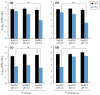
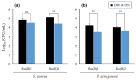
Antimicrobial photodynamic therapy (aPDT) depends on a variety of parameters notably related to the photosensitizers used, the pathogens to target and the environment to operate. In a previous study using a series of Ruthenium(II) polypyridyl ([Ru(II)]) complexes, we reported the importance of the chemical structure on both their photo-physical/physico-chemical properties and their efficacy for aPDT. By employing standard in vitro conditions, effective [Ru(II)]-mediated aPDT was demonstrated against planktonic cultures of Pseudomonas aeruginosa and Staphylococcus aureus strains notably isolated from the airways of Cystic Fibrosis (CF) patients. CF lung disease is characterized with many pathophysiological disorders that can compromise the effectiveness of antimicrobials. Taking this into account, the present study is an extension of our previous work, with the aim of further investigating [Ru(II)]-mediated aPDT under in vitro experimental settings approaching the conditions of infected airways in CF patients. Thus, we herein studied the isolated influence of a series of parameters (including increased osmotic strength, acidic pH, lower oxygen availability, artificial sputum medium and biofilm formation) on the properties of two selected [Ru(II)] complexes. Furthermore, these compounds were used to evaluate the possibility to photoinactivate P. aeruginosa while preserving an underlying epithelium of human bronchial epithelial cells. Altogether, our results provide substantial evidence for the relevance of [Ru(II)]-based aPDT in CF lung airways. Besides optimized nano-complexes, this study also highlights the various needs for translating such a challenging perspective into clinical practice.
Keywords: antimicrobial photodynamic therapy; antimicrobial resistance; benchmark analysis; biofilm; cystic fibrosis; micro-environment; ruthenium complexes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








References
-
- Youf R., Müller M., Balasini A., Thétiot F., Müller M., Hascoët A., Jonas U., Schönherr H., Lemercier G., Montier T., et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics. 2021;13:1995. doi: 10.3390/pharmaceutics13121995. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

