Imatinib Optimized Therapy Improves Major Molecular Response Rates in Patients with Chronic Myeloid Leukemia
- PMID: 36015302
- PMCID: PMC9414005
- DOI: 10.3390/pharmaceutics14081676
Imatinib Optimized Therapy Improves Major Molecular Response Rates in Patients with Chronic Myeloid Leukemia
Abstract
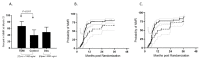
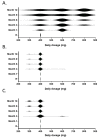
The registered dose for imatinib is 400 mg/d, despite high inter-patient variability in imatinib plasmatic exposure. Therapeutic drug monitoring (TDM) is routinely used to maximize a drug’s efficacy or tolerance. We decided to conduct a prospective randomized trial (OPTIM-imatinib trial) to assess the value of TDM in patients with chronic phase chronic myelogenous treated with imatinib as first-line therapy (NCT02896842). Eligible patients started imatinib at 400 mg daily, followed by imatinib [C]min assessment. Patients considered underdosed ([C]min < 1000 ng/mL) were randomized in a dose-increase strategy aiming to reach the threshold of 1000 ng/mL (TDM arm) versus standard imatinib management (control arm). Patients with [C]min levels ≥ 1000 ng/mL were treated following current European Leukemia Net recommendations (observational arm). The primary endpoint was the rate of major molecular response (MMR, BCR::ABL1IS ≤ 0.1%) at 12 months. Out of 133 evaluable patients on imatinib 400 mg daily, 86 patients had a [C]min < 1000 ng/mL and were randomized. The TDM strategy resulted in a significant increase in [C]min values with a mean imatinib daily dose of 603 mg daily. Patients included in the TDM arm had a 12-month MMR rate of 67% (95% CI, 51−81) compared to 39% (95% CI, 24−55) for the control arm (p = 0.017). This early advantage persisted over the 3-year study period, in which we considered imatinib cessation as a censoring event. Imatinib TDM was feasible and significantly improved the 12-month MMR rate. This early advantage may be beneficial for patients without easy access to second-line TKIs.
Keywords: chronic myelogenous leukemia; imatinib; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. - DOI - PubMed
-
- O’Brien S.G., Guilhot F., Larson R.A., Gathmann I., Baccarani M., Cervantes F., Cornelissen J.J., Fischer T., Hochhaus A., Hughes T., et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. - DOI - PubMed
-
- Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

