In Vitro and In Silico Potential Inhibitory Effects of New Biflavonoids from Ochna rhizomatosa on HIV-1 Integrase and Plasmodium falciparum
- PMID: 36015326
- PMCID: PMC9414862
- DOI: 10.3390/pharmaceutics14081701
In Vitro and In Silico Potential Inhibitory Effects of New Biflavonoids from Ochna rhizomatosa on HIV-1 Integrase and Plasmodium falciparum
Abstract
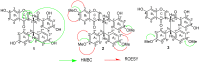
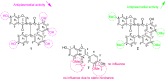
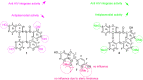
The aim of this study was to identify bioactive secondary metabolites from Ochna rhizomatosa with potential inhibitory effects against HIV and Plasmodium falciparum. A phytochemical study of O. rhizomatosa root barks resulted in the identification of three new biflavonoids (1-3), along with four known ones (4-7). Compound 7 (Gerontoisoflavone A) was a single flavonoid present in the rootbark of the plant and was used as a reference. Compound 1 (IC50 = 0.047 µM) was the only one with a noteworthy inhibitory effect against HIV-1 integrase in vitro. Chicoric acid (IC50 = 0.006 µM), a pure competitive inhibitor of HIV-1 integrase, was used as control. Compound 2 exhibited the highest antiplasmodial activity (IC50 = 4.60 µM) against the chloroquine-sensitive strain of Plasmodium falciparum NF54. Computational molecular docking revealed that compounds 1 and 2 had the highest binding score (-121.8 and -131.88 Kcal/mol, respectively) in comparison to chicoric acid and Dolutegravir (-116 and -100 Kcal/mol, respectively), towards integrase receptor (PDB:3LPT). As far as Plasmodium-6 cysteine s48/45 domain inhibition is concerned, compounds 1 and 2 showed the highest binding scores in comparison to chloroquine, urging the analysis of these compounds in vivo for disease treatment. These results confirm the potential inhibitory effect of compounds 1 and 2 for HIV and malaria treatment. Therefore, our future investigation to find inhibitors of these receptors in vivo could be an effective strategy for developing new drugs.
Keywords: HIV-1 replication; Ochna rhizomatosa; Plasmodium falciparum NF54; biflavonoids; molecular docking; structure–activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- United States Agency for International Development . USAID; USA: 2020. [(accessed on 27 June 2021)]. U.S. President’s Malaria Initiative. Available online: https://www.cdc.gov/malaria/malaria_worldwide/cdc_activities/pmi.html.
-
- World Health Organization Cameroon: Malaria Kills 3000 People in 2018. Published on 26 April 2019 at 16h21 by the Ministry of Public Health. Cameroon. 2019. [(accessed on 20 July 2022)]. Available online: https://www.journalducameroun.com/en/cameroon-malaria-kills-3000-people-....
-
- World Health Organization . WHO; Geneva: 2018. [(accessed on 20 July 2022)]. Guidelines for the Treatment of Malaria. Available online: https://www.who.int/malaria/publications/atoz/9789241549127.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

