Efficient Delivery of DNA Using Lipid Nanoparticles
- PMID: 36015328
- PMCID: PMC9416266
- DOI: 10.3390/pharmaceutics14081698
Efficient Delivery of DNA Using Lipid Nanoparticles
Abstract
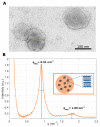
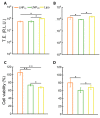
DNA vaccination has been extensively studied as a promising strategy for tumor treatment. Despite the efforts, the therapeutic efficacy of DNA vaccines has been limited by their intrinsic poor cellular internalization. Electroporation, which is based on the application of a controlled electric field to enhance DNA penetration into cells, has been the method of choice to produce acceptable levels of gene transfer in vivo. However, this method may cause cell damage or rupture, non-specific targeting, and even degradation of pDNA. Skin irritation, muscle contractions, pain, alterations in skin structure, and irreversible cell damage have been frequently reported. To overcome these limitations, in this work, we use a microfluidic platform to generate DNA-loaded lipid nanoparticles (LNPs) which are then characterized by a combination of dynamic light scattering (DLS), synchrotron small-angle X-ray scattering (SAXS), and transmission electron microscopy (TEM). Despite the clinical successes obtained by LNPs for mRNA and siRNA delivery, little is known about LNPs encapsulating bulkier DNA molecules, the clinical application of which remains challenging. For in vitro screening, LNPs were administered to human embryonic kidney 293 (HEK-293) and Chinese hamster ovary (CHO) cell lines and ranked for their transfection efficiency (TE) and cytotoxicity. The LNP formulation exhibiting the highest TE and the lowest cytotoxicity was then tested for the delivery of the DNA vaccine pVAX-hECTM targeting the human neoantigen HER2, an oncoprotein overexpressed in several cancer types. Using fluorescence-activated cell sorting (FACS), immunofluorescence assays and fluorescence confocal microscopy (FCS), we proved that pVAX-hECTM-loaded LNPs produce massive expression of the HER2 antigen on the cell membrane of HEK-293 cells. Our results provide new insights into the structure-activity relationship of DNA-loaded LNPs and pave the way for the access of this gene delivery technology to preclinical studies.
Keywords: DNA vaccines; HER2; lipid nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Vanni G., Pellicciaro M., Materazzo M., Pedini D., Portarena I., Buonomo C., Perretta T., Rizza S., Pistolese C.A., Buonomo O.C. Advanced stages and increased need for adjuvant treatments in breast cancer patients: The effect of the one-year COVID-19 pandemic. Anticancer Res. 2021;41:2689–2696. doi: 10.21873/anticanres.15050. - DOI - PubMed
-
- Vardy J.L., Liew A., Warby A., Elder A., Keshet I., Devine R., Ouliaris C., Renton C., Tattersall M.H., Dhillon H.M. On the receiving end: Have patient perceptions of the side-effects of cancer chemotherapy changed since the twentieth century? Support. Care Cancer. 2022;30:3503–3512. doi: 10.1007/s00520-022-06804-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

