A Rapid Thermal Absorption Rate and High Latent Heat Enthalpy Phase Change Fiber Derived from Bio-Based Low Melting Point Copolyesters
- PMID: 36015555
- PMCID: PMC9413292
- DOI: 10.3390/polym14163298
A Rapid Thermal Absorption Rate and High Latent Heat Enthalpy Phase Change Fiber Derived from Bio-Based Low Melting Point Copolyesters
Abstract
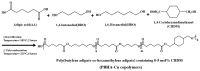
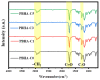
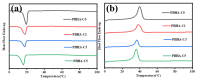
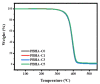
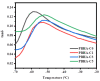
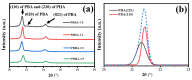
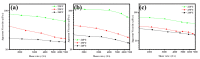
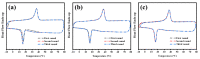
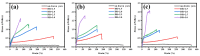
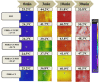
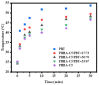
A series of poly(butylene adipate-co-hexamethylene adipate) (PBHA) copolymers with different content of 1,4-cyclohexanedimethanol (CHDM) was synthesized via one-step melt polymerization. The PBHA copolymer with 5 mol% CHDM (PBHA-C5) exhibited a low melting point (Tm) and high enthalpy of fusion (∆Hm) of 35.7 °C and 43.9 J g-1, respectively, making it a potential candidate for an ambient temperature adjustment textile phase change material (PCM). Polybutylene terephthalate (PBT) was selected as the matrix and blended at different weight ratios of PBHA-C5, and the blended samples showed comparable Tm and ∆Hm after three cycles of cooling and reheating, indicating good maintenance of their phase changing ability. Samples were then processed via melt spinning with a take-up speed of 200 m min-1 at draw ratios (DR) of 1.0 to 3.0 at 50 °C. The fiber's mechanical strength could be enhanced to 2.35 g den-1 by increasing the DR and lowering the PBHA-C5 content. Infrared thermography showed that a significant difference of more than 5 °C between PBT and other samples was achieved within 1 min of heating, indicating the ability of PBHA-C5 to adjust the temperature. After heating for 30 min, the temperatures of neat PBT, blended samples with 27, 30, and 33 wt% PBHA-C5, and neat PBHA-C5 were 53.8, 50.2, 48.3, 47.2, and 46.5 °C, respectively, and reached an equilibrium state, confirming the temperature adjustment ability of PBHA-C5 and suggesting that it can be utilized in thermoregulating applications.
Keywords: 1,4-cyclohexanedimethanol; melt spinning; phase change material; poly(butylene adipate-co-hexamethylene adipate).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yang K., Venkataraman M., Zhang X., Wiener J., Zhu G., Yao J., Militky J. Review: Incorporation of Organic PCMs into Textiles. J. Mater. Sci. 2022;57:798–847. doi: 10.1007/s10853-021-06641-3. - DOI
-
- Zhang N., Yuan Y., Cao X., Du Y., Zhang Z., Gui Y. Latent Heat Thermal Energy Storage Systems with Solid-Liquid Phase Change Materials: A Review. Adv. Eng. Mater. 2018;20:1700753. doi: 10.1002/adem.201700753. - DOI
-
- Alva G., Lin Y., Liu L., Fang G. Synthesis, Characterization and Applications of Microencapsulated Phase Change Materials in Thermal Energy Storage: A Review. Energy Build. 2017;144:276–294. doi: 10.1016/j.enbuild.2017.03.063. - DOI
-
- Chalco-Sandoval W., Fabra M.J., López-Rubio A., Lagaron J.M. Use of Phase Change Materials to Develop Electrospun Coatings of Interest in Food Packaging Applications. J. Food Eng. 2017;192:122–128. doi: 10.1016/j.jfoodeng.2015.01.019. - DOI
-
- Zhu C., Chen Y., Cong R., Ran F., Fang G. Improved Thermal Properties of Stearic Acid/High Density Polyethylene/Carbon Fiber Composite Heat Storage Materials. Sol. Energy Mater. Sol. Cells. 2021;219:110782. doi: 10.1016/j.solmat.2020.110782. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

