Overview of Tools and Measures Investigating Vaccine Hesitancy in a Ten Year Period: A Scoping Review
- PMID: 36016086
- PMCID: PMC9412526
- DOI: 10.3390/vaccines10081198
Overview of Tools and Measures Investigating Vaccine Hesitancy in a Ten Year Period: A Scoping Review
Abstract
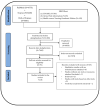
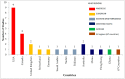
The challenge of vaccine hesitancy, a growing global concern in the last decade, has been aggravated by the COVID-19 pandemic. The need for monitoring vaccine sentiments and early detection of vaccine hesitancy in a population recommended by the WHO calls for the availability of contextually relevant tools and measures. This scoping review covers a ten year-period from 2010-2019 which included the first nine years of the decade of vaccines and aims to give a broad overview of tools and measures, and present a summary of their nature, similarities, and differences. We conducted the review using the framework for scoping reviews by Arksey and O'Malley (2005) and reported it following the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews' guidelines. Of the 26 studies included, only one was conducted in the WHO African Region. Measures for routine childhood vaccines were found to be the most preponderant in the reviewed literature. The need for validated, contextually relevant tools in the WHO Africa Region is essential, and made more so by the scourge of the ongoing pandemic in which vaccination is critical for curtailment.
Keywords: immunization; measures; scoping review; tools; vaccination; vaccine hesitancy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Stratford J., MacKenzie E., Mockford E. Balancing Speed and Safety: The Authorisation of COVID-19 Vaccines and Medicines. Judic. Rev. 2020;25:105–117. doi: 10.1080/10854681.2020.1780664. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources