In Vitro Evaluation and Mitigation of Niclosamide's Liabilities as a COVID-19 Treatment
- PMID: 36016172
- PMCID: PMC9412300
- DOI: 10.3390/vaccines10081284
In Vitro Evaluation and Mitigation of Niclosamide's Liabilities as a COVID-19 Treatment
Abstract
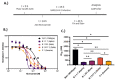
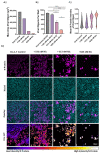
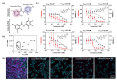
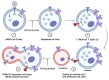
Niclosamide, an FDA-approved oral anthelmintic drug, has broad biological activity including anticancer, antibacterial, and antiviral properties. Niclosamide has also been identified as a potent inhibitor of SARS-CoV-2 infection in vitro, generating interest in its use for the treatment or prevention of COVID-19. Unfortunately, there are several potential issues with using niclosamide for COVID-19, including low bioavailability, significant polypharmacology, high cellular toxicity, and unknown efficacy against emerging SARS-CoV-2 variants of concern. In this study, we used high-content imaging-based immunofluorescence assays in two different cell models to assess these limitations and evaluate the potential for using niclosamide as a COVID-19 antiviral. We show that despite promising preliminary reports, the antiviral efficacy of niclosamide overlaps with its cytotoxicity giving it a poor in vitro selectivity index for anti-SARS-CoV-2 inhibition. We also show that niclosamide has significantly variable potency against the different SARS-CoV-2 variants of concern and is most potent against variants with enhanced cell-to-cell spread including the B.1.1.7 (alpha) variant. Finally, we report the activity of 33 niclosamide analogs, several of which have reduced cytotoxicity and increased potency relative to niclosamide. A preliminary structure-activity relationship analysis reveals dependence on a protonophore for antiviral efficacy, which implicates nonspecific endolysosomal neutralization as a dominant mechanism of action. Further single-cell morphological profiling suggests niclosamide also inhibits viral entry and cell-to-cell spread by syncytia. Altogether, our results suggest that niclosamide is not an ideal candidate for the treatment of COVID-19, but that there is potential for developing improved analogs with higher clinical translational potential in the future.
Keywords: COVID-19; SARS-CoV-2; drug repurposing; niclosamide; polypharmacology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Update of
-
In Vitro Evaluation and Mitigation of Niclosamide's Liabilities as a COVID-19 Treatment.bioRxiv [Preprint]. 2022 Jul 13:2022.06.24.497526. doi: 10.1101/2022.06.24.497526. bioRxiv. 2022. Update in: Vaccines (Basel). 2022 Aug 09;10(8):1284. doi: 10.3390/vaccines10081284. PMID: 35860224 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

