Evaluation of the Immunomodulatory Effect of the Recombinant 14-3-3 and Major Antigen Proteins of Strongyloides stercoralis against an Infection by S. venezuelensis
- PMID: 36016178
- PMCID: PMC9415175
- DOI: 10.3390/vaccines10081292
Evaluation of the Immunomodulatory Effect of the Recombinant 14-3-3 and Major Antigen Proteins of Strongyloides stercoralis against an Infection by S. venezuelensis
Abstract
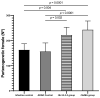
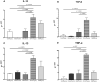
Strongyloidiasis, caused by Strongyloides stercoralis, is a neglected parasitic disease that represents a serious public health problem. In immunocompromised patients, this parasitosis can result in hyperinfection or disseminated disease with high levels of mortality. In previous studies, the mRNAs encoding for the 14-3-3 and major antigen proteins were found to be expressed at high levels in S. stercoralis L3 larvae, suggesting potential key roles in parasite-host interactions. We have produced them as recombinant proteins (rSs14-3-3 and rSsMA) in a bacterial protein expression system. The serum levels of anti-rSs14-3-3 and anti-rSsMA IgGs are increased upon infection with S. venezuelensis, validating the use of the mouse model since the native 14-3-3 and MA proteins induce an immune response. Each recombinant protein was formulated in the adjuvant adaptation (ADAD) vaccination system and injected twice, subcutaneously, in CD1 mice that were experimentally infected with 3000 S. venezuelensis L3 to evaluate their protective and immunomodulatory activity. Our results, including the number of parthenogenetic females, number of eggs in stool samples and the analysis of the splenic and intestinal indexes, show that the vaccines did not protect against infection. The immunization with rSs14-3-3 induced changes in the cytokine profile in mice, producing higher expression of IL-10, TGF-β, IL-13 and TNF-α in the spleen, suggesting a Th2/Treg-type response with an increase in TNF-α levels, confirming its role as an immunomodulator.
Keywords: 14-3-3; ADAD; Strongyloides stercoralis; cytokines; major antigen; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




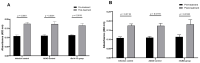
Similar articles
-
Immunoblotting using Strongyloides venezuelensis larvae, parthenogenetic females or eggs extracts for the diagnosis of experimentally infected immunosuppressed rats.Exp Parasitol. 2015 Oct;157:117-23. doi: 10.1016/j.exppara.2015.07.009. Epub 2015 Jul 26. Exp Parasitol. 2015. PMID: 26219202
-
Strongyloides venezuelensis infection augments arterial blood pressure in male wistar rats.Acta Trop. 2019 Feb;190:350-355. doi: 10.1016/j.actatropica.2018.12.007. Epub 2018 Dec 6. Acta Trop. 2019. PMID: 30529092
-
Previous contact with Strongyloides venezuelensis contributed to prevent insulitis in MLD-STZ diabetes.Exp Parasitol. 2013 Jun;134(2):183-9. doi: 10.1016/j.exppara.2013.03.007. Epub 2013 Mar 20. Exp Parasitol. 2013. PMID: 23523576
-
Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis.Parasite Immunol. 2004 Nov-Dec;26(11-12):487-97. doi: 10.1111/j.0141-9838.2004.00726.x. Parasite Immunol. 2004. PMID: 15771684 Review.
-
Strongyloides infection in rodents: immune response and immune regulation.Parasitology. 2017 Mar;144(3):295-315. doi: 10.1017/S0031182016000111. Epub 2016 Feb 24. Parasitology. 2017. PMID: 26905057 Review.
References
-
- World Health Organization Soil-Transmitted Helminth Infections. Newsroom. Jan 10, 2022. [(accessed on 1 June 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helmin....
-
- Aramendia A.A., Anegagrie M., Zewdie D., Dacal E., Saugar J.M., Herrador Z., Hailu T., Yimer M., Periago M.V., Rodriguez E., et al. Epidemiology of intestinal helminthiases in a rural community of Ethiopia: Is it time to expand control programs to include Strongyloides stercoralis and the entire community? PLoS Negl. Trop. Dis. 2020;14:e0008315. doi: 10.1371/journal.pntd.0008315. - DOI - PMC - PubMed
-
- Botero D., Restrepo M. Parasitosis Humanas. 5th ed. Corporación para Investigaciones Biológicas; Medellín, Colombia: 2012. pp. 162–177.
-
- Igual Adell R., Domínguez Márquez V. Estrongiloidiasis: Epidemiología, manifestaciones clínicas y diagnóstico. Experiencia en una zona endémica: La comarca de La Safor (Valencia) Enferm. Infecc. Microb. Clin. 2007;25:38–44. doi: 10.1157/13111836. - DOI
Grants and funding
- Juan Castelló doctoral scholarship/Valencia City Council-Universitat de València
- RICET RD16/0027/0018; PI19/01727; RTI2018-099474-B-I00; PID2019-105713GB-I00/AEI/10.13039/501100011033/State Research Agency, Ministry of Science and Innovation, Spain
- PROMETEO/2020/071/Department of Education, Culture and Sports, Generalitat Valenciana, Valencia, Spain
LinkOut - more resources
Full Text Sources

