Improved SARS-CoV-2 Neutralization of Delta and Omicron BA.1 Variants of Concern after Fourth Vaccination in Hemodialysis Patients
- PMID: 36016216
- PMCID: PMC9415993
- DOI: 10.3390/vaccines10081328
Improved SARS-CoV-2 Neutralization of Delta and Omicron BA.1 Variants of Concern after Fourth Vaccination in Hemodialysis Patients
Abstract
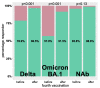
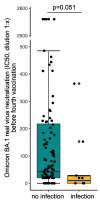
Hemodialysis patients are exposed to a markedly increased risk when infected with SARS-CoV-2. To date, it is unclear if hemodialysis patients benefit from four vaccinations. A total of 142 hemodialysis patients received four COVID-19 vaccinations until March 2022. RDB binding antibody titers were determined in a competitive surrogate neutralization assay. Vero-E6 cells were infected with SARS-CoV-2 variants of concern (VoC), Delta (B.1.617.2), or Omicron (B.1.1.529, sub-lineage BA.1) to determine serum infection neutralization capacity. Four weeks after the fourth vaccination, serum infection neutralization capacity significantly increased from a 50% inhibitory concentration (IC50, serum dilution factor 1:x) of 247.0 (46.3−1560.8) to 2560.0 (1174.0−2560.0) for the Delta VoC, and from 37.5 (20.0−198.8) to 668.5 (182.2−2560.0) for the Omicron VoC (each p < 0.001) compared to four months after the third vaccination. A significant increase in the neutralization capacity was even observed for patients with high antibody titers after three vaccinations (p < 0.001). Ten patients with SARS-CoV-2 breakthrough infection after the first blood sampling had by trend lower prior neutralization capacity for Omicron (p = 0.051). Our findings suggest that hemodialysis patients benefit from a fourth vaccination in particular in the light of the highly infectious SARS-CoV-2 Omicron-variants. A routinely applied four-time vaccination seems to broaden immunity against variants and would be recommended in hemodialysis patients.
Keywords: COVID-19 vaccination; SARS-CoV-2; hemodialysis; in-vitro viral neutralization.
Conflict of interest statement
The authors declare no conflict of interest. UP is receiving grants from Hoehnle AG, SCG Cell Therapy and VirBio and personal fees from Abbott, Abbvie, Arbutus, Gilead, GSK, J&J, Roche, Sanofi, Sobi and Vaccitech. UP is co-founder and share-holder of SCG Cell Therapy.
Figures


References
-
- Hilbrands L.B., Duivenvoorden R., Vart P., Franssen C.F.M., Hemmelder M.H., Jager K.J., Kieneker L.M., Noordzij M., Pena M.J., Vries H., et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. - DOI - PMC - PubMed
-
- Erber J., Kappler V., Haller B., Mijočević H., Galhoz A., Prazeres da Costa C., Gebhardt F., Graf N., Hoffmann D., Thaler M., et al. Infection Control Measures and Prevalence of SARS-CoV-2 IgG among 4,554 University Hospital Employees, Munich, Germany. Emerg. Infect. Dis. 2022;28:572–581. doi: 10.3201/eid2803.204436. - DOI - PMC - PubMed
-
- Espi M., Charmetant X., Barba T., Mathieu C., Pelletier C., Koppe L., Chalencon E., Kalbacher E., Mathias V., Ovize A., et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022;101:390–402. doi: 10.1016/j.kint.2021.10.040. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

