Ivermectin Inhibits the Replication of Usutu Virus In Vitro
- PMID: 36016263
- PMCID: PMC9413757
- DOI: 10.3390/v14081641
Ivermectin Inhibits the Replication of Usutu Virus In Vitro
Abstract
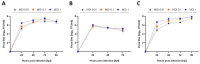
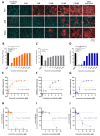
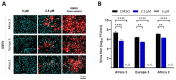
Usutu virus (USUV) is an emerging mosquito-borne arbovirus within the genus Flavivirus, family Flaviviridae. Similar to the closely related West Nile virus (WNV), USUV infections are capable of causing mass mortality in wild and captive birds, especially blackbirds. In the last few years, a massive spread of USUV was present in the avian population of Germany and other European countries. To date, no specific antiviral therapies are available. Nine different approved drugs were tested for their antiviral effects on the replication of USUV in vitro in a screening assay. Ivermectin was identified as a potent inhibitor of USUV replication in three cell types from different species, such as simian Vero CCL-81, human A549 and avian TME R. A 2- to 7-log10 reduction of the viral titer in the supernatant was detected at a non-cytotoxic concentration of 5 µM ivermectin dependent on the applied cell line. IC50 values of ivermectin against USUV lineage Africa 3 was found to be 0.55 µM in Vero CCL-81, 1.94 µM in A549 and 1.38 µM in TME-R cells. The antiviral efficacy was comparable between the USUV lineages Africa 2, Africa 3 and Europe 3. These findings show that ivermectin may be a candidate for further experimental and clinical studies addressing the treatment of USUV disease, especially in captive birds.
Keywords: Usutu virus; antiviral; bird; drug; ivermectin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Steinmetz H.W., Bakonyi T., Weissenböck H., Hatt J.-M., Eulenberger U., Robert N., Hoop R., Nowotny N. Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland-genomic and pathologic comparison to other central European outbreaks. Vet. Microbiol. 2011;148:207–212. doi: 10.1016/j.vetmic.2010.09.018. - DOI - PubMed
-
- Cadar D., Lühken R., van der Jeugd H., Garigliany M., Ziegler U., Keller M., Lahoreau J., Lachmann L., Becker N., Kik M., et al. Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Eurosurveillance. 2017;22:30452. doi: 10.2807/1560-7917.ES.2017.22.4.30452. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

