Rotavirus Strain Trends in United States, 2009-2016: Results from the National Rotavirus Strain Surveillance System (NRSSS)
- PMID: 36016397
- PMCID: PMC9414880
- DOI: 10.3390/v14081775
Rotavirus Strain Trends in United States, 2009-2016: Results from the National Rotavirus Strain Surveillance System (NRSSS)
Abstract
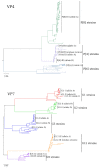
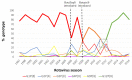
Before the introduction of vaccines, group A rotaviruses (RVA) were the leading cause of acute gastroenteritis in children worldwide. The National Rotavirus Strain Surveillance System (NRSSS) was established in 1996 by the Centers for Disease Control and Prevention (CDC) to perform passive RVA surveillance in the USA. We report the distribution of RVA genotypes collected through NRSSS during the 2009-2016 RVA seasons and retrospectively examine the genotypes detected through the NRSSS since 1996. During the 2009-2016 RVA seasons, 2134 RVA-positive fecal specimens were sent to the CDC for analysis of the VP7 and VP4 genes by RT-PCR genotyping assays and sequencing. During 2009-2011, RVA genotype G3P[8] dominated, while G12P[8] was the dominant genotype during 2012-2016. Vaccine strains were detected in 1.7% of specimens and uncommon/unusual strains, including equine-like G3P[8] strains, were found in 1.9%. Phylogenetic analyses showed limited VP7 and VP4 sequence variation within the common genotypes with 1-3 alleles/lineages identified per genotype. A review of 20 years of NRSSS surveillance showed two changes in genotype dominance, from G1P[8] to G3P[8] and then G3P[8] to G12P[8]. A better understanding of the long-term effects of vaccine use on epidemiological and evolutionary dynamics of circulating RVA strains requires continued surveillance.
Keywords: RVA; genotype; prevalence; rotavirus; surveillance; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cortese M.M., Parashar U.D. Prevention of rotavirus gastroenteritis among infants and children: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2009;58:1–25. - PubMed
-
- Parashar U.D., Alexander J.P., Glass R.I. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2006;55:1–13. - PubMed
-
- Zhen S.S., Li Y., Wang S.M., Zhang X.J., Hao Z.Y., Chen Y., Wang D., Zhang Y.H., Zhang Z.Y., Ma J.C., et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerg. Microbes Infect. 2015;4:e64. doi: 10.1038/emi.2015.64. - DOI - PMC - PubMed
-
- Dang D.A., Nguyen V.T., Vu D.T., Nguyen T.H., Nguyen D.M., Yuhuan W., Baoming J., Nguyen D.H., Le T.L., Rotavin M.V.T.G. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine. 2012;30:A114–A121. doi: 10.1016/j.vaccine.2011.07.118. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

