Porcine Enteric Coronavirus PEDV Induces the ROS-ATM and Caspase7-CAD-γH2AX Signaling Pathways to Foster Its Replication
- PMID: 36016404
- PMCID: PMC9413700
- DOI: 10.3390/v14081782
Porcine Enteric Coronavirus PEDV Induces the ROS-ATM and Caspase7-CAD-γH2AX Signaling Pathways to Foster Its Replication
Abstract
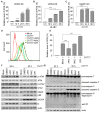
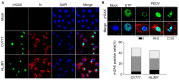
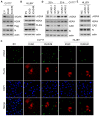
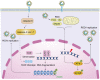
DNA damage response (DDR) is an evolutionarily conserved mechanism by which eukaryotic cells sense DNA lesions caused by intrinsic and extrinsic stimuli, including virus infection. Although interactions between DNA viruses and DDR have been extensively studied, how RNA viruses, especially coronaviruses, regulate DDR remains unknown. A previous study showed that the porcine epidemic diarrhea virus (PEDV), a member of the genus Alphacoronavirus in the Coronaviridae family, induces DDR in infected cells. However, the underlying mechanism was unclear. This study showed that PEDV activates the ATM-Chk2 signaling, while inhibition of ATM or Chk2 dampens the early stage of PEDV infection. Additionally, we found that PEDV-activated ATM signaling correlates with intracellular ROS production. Interestingly, we showed that, unlike the typical γH2AX foci, PEDV infection leads to a unique γH2AX staining pattern, including phase I (nuclear ring staining), II (pan-nuclear staining), and III (co-staining with apoptotic bodies), which highly resembles the apoptosis process. Furthermore, we demonstrated that PEDV-induced H2AX phosphorylation depends on the activation of caspase-7 and caspase-activated DNAse (CAD), but not ATM-Chk2. Finally, we showed that the knockdown of H2AX attenuates PEDV replication. Taken together, we conclude that PEDV induces DDR through the ROS-ATM and caspase7-CAD-γH2AX signaling pathways to foster its early replication.
Keywords: ATM; DNA damage response; PEDV; caspase; γH2AX.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Coronavirus Endoribonuclease Activity in Porcine Epidemic Diarrhea Virus Suppresses Type I and Type III Interferon Responses.J Virol. 2019 Apr 3;93(8):e02000-18. doi: 10.1128/JVI.02000-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30728254 Free PMC article.
-
JNK and p38 mitogen-activated protein kinase pathways contribute to porcine epidemic diarrhea virus infection.Virus Res. 2016 Aug 15;222:1-12. doi: 10.1016/j.virusres.2016.05.018. Epub 2016 May 20. Virus Res. 2016. PMID: 27215486 Free PMC article.
-
Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling.J Virol. 2018 Jan 30;92(4):e01677-17. doi: 10.1128/JVI.01677-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29187542 Free PMC article.
-
Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control.Virus Res. 2020 Sep;286:198045. doi: 10.1016/j.virusres.2020.198045. Epub 2020 Jun 2. Virus Res. 2020. PMID: 32502552 Free PMC article. Review.
-
Epidemiology of porcine epidemic diarrhea virus among Chinese pig populations: A meta-analysis.Microb Pathog. 2019 Apr;129:43-49. doi: 10.1016/j.micpath.2019.01.017. Epub 2019 Jan 23. Microb Pathog. 2019. PMID: 30682525
Cited by
-
Progress on innate immune evasion and live attenuated vaccine of pseudorabies virus.Front Microbiol. 2023 Mar 3;14:1138016. doi: 10.3389/fmicb.2023.1138016. eCollection 2023. Front Microbiol. 2023. PMID: 36937252 Free PMC article. Review.
-
HDAC-Specific Inhibitors Induce the Release of Porcine Epidemic Diarrhea Virus via the COPII-Coated Vesicles.Viruses. 2023 Sep 4;15(9):1874. doi: 10.3390/v15091874. Viruses. 2023. PMID: 37766280 Free PMC article.
-
For better or worse: crosstalk of parvovirus and host DNA damage response.Front Immunol. 2024 Feb 23;15:1324531. doi: 10.3389/fimmu.2024.1324531. eCollection 2024. Front Immunol. 2024. PMID: 38464523 Free PMC article. Review.
-
RSL3 Inhibits Porcine Epidemic Diarrhea Virus Replication by Activating Ferroptosis.Viruses. 2023 Oct 12;15(10):2080. doi: 10.3390/v15102080. Viruses. 2023. PMID: 37896857 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

