A Systematic Review and Meta-Analysis of Serologic Response following Coronavirus Disease 2019 (COVID-19) Vaccination in Solid Organ Transplant Recipients
- PMID: 36016444
- PMCID: PMC9413038
- DOI: 10.3390/v14081822
A Systematic Review and Meta-Analysis of Serologic Response following Coronavirus Disease 2019 (COVID-19) Vaccination in Solid Organ Transplant Recipients
Abstract
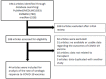
Solid organ transplant (SOT) recipients are at greater risk of coronavirus disease 2019 (COVID-19) and have attenuated response to vaccinations. In the present meta-analysis, we aimed to evaluate the serologic response to the COVID-19 vaccine in SOT recipients. A search of electronic databases was conducted to identify SOT studies that reported the serologic response to COVID-19 vaccination. We analyzed 44 observational studies including 6158 SOT recipients. Most studies were on mRNA vaccination (mRNA-1273 or BNT162b2). After a single and two doses of vaccine, serologic response rates were 8.6% (95% CI 6.8-11.0) and 34.2% (95% CI 30.1-38.7), respectively. Compared to controls, response rates were lower after a single and two doses of vaccine (OR 0.0049 [95% CI 0.0021-0.012] and 0.0057 [95% CI 0.0030-0.011], respectively). A third dose improved the rate to 65.6% (95% CI 60.4-70.2), but in a subset of patients who had not achieved a response after two doses, it remained low at 35.7% (95% CI 21.2-53.3). In summary, only a small proportion of SOT recipients achieved serologic response to the COVID-19 mRNA vaccine, and that even the third dose had an insufficient response. Alternative strategies for prophylaxis in SOT patients need to be developed. Key Contribution: In this meta-analysis that included 6158 solid organ transplant recipients, the serologic response to the COVID-19 vaccine was extremely low after one (8.6%) and two doses (34.2%). The third dose of the vaccine improved the rate only to 66%, and in the subset of patients who had not achieved a response after two doses, it remained low at 36%. The results of our study suggest that a significant proportion of solid organ transplant recipients are unable to achieve a sufficient serologic response after completing not only the two series of vaccination but also the third booster dose. There is an urgent need to develop strategies for prophylaxis including modified vaccine schedules or the use of monoclonal antibodies in this vulnerable patient population.
Keywords: COVID-19; heart transplant; immunocompromised; kidney transplant; lung transplant; meta-analysis; outcomes; solid organ transplant; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
-
- Salto-Alejandre S., Jimenez-Jorge S., Sabe N., Ramos-Martinez A., Linares L., Valerio M., Martin-Davila P., Fernandez-Ruiz M., Farinas M.C., Blanes-Julia M., et al. Risk factors for unfavorable outcome and impact of early post-transplant infection in solid organ recipients with COVID-19: A prospective multicenter cohort study. PLoS ONE. 2021;16:e0250796. doi: 10.1371/journal.pone.0250796. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

