Efficacy of Difelikefalin for the Treatment of Moderate to Severe Pruritus in Hemodialysis Patients: Pooled Analysis of KALM-1 and KALM-2 Phase 3 Studies
- PMID: 36016762
- PMCID: PMC9396406
- DOI: 10.1016/j.xkme.2022.100512
Efficacy of Difelikefalin for the Treatment of Moderate to Severe Pruritus in Hemodialysis Patients: Pooled Analysis of KALM-1 and KALM-2 Phase 3 Studies
Abstract
Rationale & objective: Chronic kidney disease-associated pruritus (CKD-aP) in patients treated by hemodialysis (HD) impairs quality of life (QoL). Difelikefalin, a selective κ-opioid receptor agonist, decreased the intensity of CKD-aP in patients undergoing HD. This pooled analysis evaluated difelikefalin's efficacy and the itch-related QoL overall and in subgroups defined by demographics or disease characteristics.
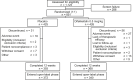
Study design: In KALM-1 and KALM-2, participants were randomized (1:1) to receive intravenous difelikefalin or placebo 3 times/wk for 12 weeks, followed by a 52-week open-label extension.
Setting & participants: Adults with moderate to severe CKD-aP treated by HD in North America, Europe, and the Asia-Pacific region.
Intervention: Intravenous difelikefalin at 0.5 mcg/kg or placebo.
Outcomes: Itch intensity (Worst Itching Intensity Numerical Rating Scale [WI-NRS]) and itch-related QoL (Skindex-10 and 5-D Itch questionnaires).
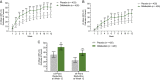
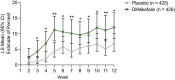
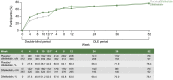
Results: 851 participants were randomized (difelikefalin, n = 426; placebo, n = 425). This pooled analysis demonstrated early (week 1), sustained difelikefalin efficacy, with significantly greater achievement of ≥3-point WI-NRS reduction with difelikefalin (51.1%) versus placebo (35.2%; P < 0.001). Achievement of a ≥4-point WI-NRS reduction was significantly greater with difelikefalin (38.7%) versus placebo (23.4%; P < 0.001). Difelikefalin reduced itch intensity in subgroups based on age, sex, anti-itch medication use, the presence of specific medical conditions, and gabapentin or pregabalin use. More participants receiving difelikefalin versus placebo achieved clinically meaningful decreases of ≥15 points on the Skindex-10 scale (55.5% vs 40.5%, respectively; P < 0.001) and ≥5 points on the 5-D Itch scale (52.1% vs 42.3%, respectively; P = 0.01), with sustained 5-D Itch effects up to 64 weeks.
Limitations: Subgroup samples were small. The WI-NRS, Skindex-10, and 5-D Itch are not used in routine clinical care of dialysis patients; therefore, findings may not reflect the real-world effectiveness of difelikefalin.
Conclusions: Difelikefalin demonstrated rapid, sustained efficacy, with consistent results in diverse populations of patients treated by HD.
Funding: Cara Therapeutics, Inc.
Trial registration: The KALM-1 trial is registered as NCT03422653 and the KALM-2 trial is registered as NCT03636269.
Keywords: Chronic kidney disease; difelikefalin; efficacy; pruritus; κ-opioid receptor.
© 2022 The Authors.
Figures



References
-
- Mettang T., Kremer A.E. Uremic pruritus. Kidney Int. 2015;87(4):685–691. - PubMed
-
- Pisoni R.L., Wikström B., Elder S.J., et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21(12):3495–3505. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

