Advances in the use of exosomes for the treatment of ALI/ARDS
- PMID: 36016948
- PMCID: PMC9396740
- DOI: 10.3389/fimmu.2022.971189
Advances in the use of exosomes for the treatment of ALI/ARDS
Abstract
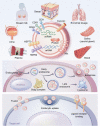
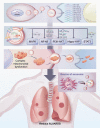
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) is a critical clinical syndrome with high morbidity and mortality. Currently, the primary treatment for ALI/ARDS is mainly symptomatic therapy such as mechanical ventilation and fluid management. Due to the lack of effective treatment strategies, most ALI/ARDS patients face a poor prognosis. The discovery of exosomes has created a promising prospect for the treatment of ALI/ARDS. Exosomes can exert anti-inflammatory effects, inhibit apoptosis, and promote cell regeneration. The microRNA contained in exosomes can participate in intercellular communication and play an immunomodulatory role in ALI/ARDS disease models. This review discusses the possible mechanisms of exosomes in ALI/ARDS to facilitate the development of innovative treatments for ALI/ARDS.
Keywords: acute lung injury; acute respiratory distress syndrome; exosomes; inflammation; treatment.
Copyright © 2022 Liu, Xiao and Xie.
Conflict of interest statement
The authors declare that the study was conducted without any commercial or financial relationships that could be interpreted as potential conflicts of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

