Immune-regulating camouflaged nanoplatforms: A promising strategy to improve cancer nano-immunotherapy
- PMID: 36017071
- PMCID: PMC9382433
- DOI: 10.1016/j.bioactmat.2022.07.023
Immune-regulating camouflaged nanoplatforms: A promising strategy to improve cancer nano-immunotherapy
Abstract
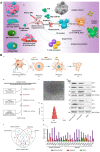
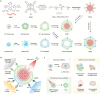
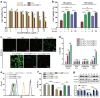
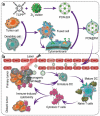
Although nano-immunotherapy has advanced dramatically in recent times, there remain two significant hurdles related to immune systems in cancer treatment, such as (namely) inevitable immune elimination of nanoplatforms and severely immunosuppressive microenvironment with low immunogenicity, hampering the performance of nanomedicines. To address these issues, several immune-regulating camouflaged nanocomposites have emerged as prevailing strategies due to their unique characteristics and specific functionalities. In this review, we emphasize the composition, performances, and mechanisms of various immune-regulating camouflaged nanoplatforms, including polymer-coated, cell membrane-camouflaged, and exosome-based nanoplatforms to evade the immune clearance of nanoplatforms or upregulate the immune function against the tumor. Further, we discuss the applications of these immune-regulating camouflaged nanoplatforms in directly boosting cancer immunotherapy and some immunogenic cell death-inducing immunotherapeutic modalities, such as chemotherapy, photothermal therapy, and reactive oxygen species-mediated immunotherapies, highlighting the current progress and recent advancements. Finally, we conclude the article with interesting perspectives, suggesting future tendencies of these innovative camouflaged constructs towards their translation pipeline.
Keywords: Biological camouflage; Immune-regulating; Immunogenic cell death; Nanovaccine; Prolonged blood circulation.
© 2022 The Authors.
Conflict of interest statement
We clarify that this publication has no such conflicts of interest from either of the authors.
Figures








Similar articles
-
Engineering nanomedicines for immunogenic eradication of cancer cells: Recent trends and synergistic approaches.Acta Pharm Sin B. 2024 Jun;14(6):2475-2504. doi: 10.1016/j.apsb.2024.03.022. Epub 2024 Mar 20. Acta Pharm Sin B. 2024. PMID: 38828160 Free PMC article. Review.
-
Hybrid Cell Membrane-Based Nanoplatforms for Enhanced Immunotherapy against Cancer and Infectious Diseases.Adv Healthc Mater. 2024 Jul;13(19):e2304477. doi: 10.1002/adhm.202304477. Epub 2024 May 11. Adv Healthc Mater. 2024. PMID: 38709914 Review.
-
Nanomedicines for an Enhanced Immunogenic Cell Death-Based In Situ Cancer Vaccination Response.Acc Chem Res. 2024 Mar 19;57(6):905-918. doi: 10.1021/acs.accounts.3c00771. Epub 2024 Feb 28. Acc Chem Res. 2024. PMID: 38417027
-
Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy.Acta Pharmacol Sin. 2020 Jul;41(7):986-994. doi: 10.1038/s41401-020-0400-z. Epub 2020 Apr 21. Acta Pharmacol Sin. 2020. PMID: 32317755 Free PMC article. Review.
-
Cell membrane cloaked nanomedicines for bio-imaging and immunotherapy of cancer: Improved pharmacokinetics, cell internalization and anticancer efficacy.J Control Release. 2021 Jul 10;335:130-157. doi: 10.1016/j.jconrel.2021.05.018. Epub 2021 May 17. J Control Release. 2021. PMID: 34015400 Review.
Cited by
-
Nanoparticle-Based Drug Delivery Systems Targeting Tumor Microenvironment for Cancer Immunotherapy Resistance: Current Advances and Applications.Pharmaceutics. 2022 Sep 21;14(10):1990. doi: 10.3390/pharmaceutics14101990. Pharmaceutics. 2022. PMID: 36297426 Free PMC article. Review.
-
Immunogenic cell death: A new strategy to enhancing cancer immunotherapy.Hum Vaccin Immunother. 2024 Dec 31;20(1):2437918. doi: 10.1080/21645515.2024.2437918. Epub 2024 Dec 10. Hum Vaccin Immunother. 2024. PMID: 39655738 Free PMC article. Review.
-
Immunopharmacology of gastric cancer-deciphering immune cell subset responses and nanoparticle-mediated targeting.Front Pharmacol. 2025 May 19;16:1611234. doi: 10.3389/fphar.2025.1611234. eCollection 2025. Front Pharmacol. 2025. PMID: 40458806 Free PMC article. Review.
-
Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury.Bioact Mater. 2023 Mar 23;27:1-14. doi: 10.1016/j.bioactmat.2023.03.007. eCollection 2023 Sep. Bioact Mater. 2023. PMID: 37006825 Free PMC article.
-
The evolution of integrated magnetic hyperthermia and chemodynamic therapy for combating cancer: a comprehensive viewpoint.Nanoscale Adv. 2025 Jul 23;7(16):4820-4836. doi: 10.1039/d4na01004c. eCollection 2025 Aug 5. Nanoscale Adv. 2025. PMID: 40708892 Free PMC article. Review.
References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021, CA-Cancer. J. Clin. 2021;71(1):7–33. - PubMed
-
- Zhao Y., Chen B.Q., Kankala R.K., Wang S.B., Chen A.Z. Recent advances in combination of copper chalcogenide-based photothermal and reactive oxygen species-related therapies. ACS Biomater. Sci. Eng. 2020;6(9):4799–4815. - PubMed
-
- Yang R., Xu J., Xu L., Sun X., Chen Q., Zhao Y., Peng R., Liu Z. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12(6):5121–5129. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

