Simvastatin-hydroxyapatite coatings prevent biofilm formation and improve bone formation in implant-associated infections
- PMID: 36017072
- PMCID: PMC9395756
- DOI: 10.1016/j.bioactmat.2022.07.028
Simvastatin-hydroxyapatite coatings prevent biofilm formation and improve bone formation in implant-associated infections
Abstract
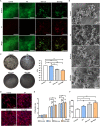
Implant-associated infections (IAIs) caused by biofilm formation are the most devastating complications of orthopedic surgery. Statins have been commonly and safely used drugs for hypercholesterolemia for many years. Here, we report that simvastatin-hydroxyapatite-coated titanium alloy prevents biofilm-associated infections. The antibacterial properties of simvastatin against Staphylococcus aureus and Staphylococcus epidermidis biofilms in vitro was confirmed by crystal violet staining and live-dead bacterial staining. We developed a simvastatin-and hydroxyapatite (Sim-HA)-coated titanium alloy via electrochemical deposition. Sim-HA coatings inhibited Staphylococcus aureus biofilm formation and improved the biocompatibility of the titanium alloy. Sim-HA coatings effectively prevented Staphylococcus aureus IAI in rat femurs, as confirmed by radiological assessment and histological examination. The antibacterial effects of the Sim-HA coatings were attributed to their inhibitory effects on biofilm formation, as verified by scanning electron microscopic observations and bacterial spread plate analysis. In addition, the Sim-HA coatings enhanced osteogenesis and osteointegration, as verified by micro-CT, histological evaluation, and biomechanical pull-out tests. In summary, Sim-HA coatings are promising implant materials for protection against biofilm-associated infections.
Keywords: Antibacterial; Biofilm; Implant-associated infections; Osteogenesis; Simvastatin.
© 2022 The Authors.
Conflict of interest statement
None.
Figures






References
-
- Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014;276(2):111–119. - PubMed
-
- Kapadia B.H., Berg R.A., Daley J.A., Fritz J., Bhave A., Mont M.A. Periprosthetic joint infection. Lancet. 2016;387(10016):386–394. - PubMed
-
- Zimmerli W., Sendi P. Orthopaedic biofilm infections. Apmis. 2017;125(4):353–364. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

