Adult health and transition stage-specific rotenone-mediated Drosophila model of Parkinson's disease: Impact on late-onset neurodegenerative disease models
- PMID: 36017079
- PMCID: PMC9398202
- DOI: 10.3389/fnmol.2022.896183
Adult health and transition stage-specific rotenone-mediated Drosophila model of Parkinson's disease: Impact on late-onset neurodegenerative disease models
Abstract
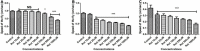
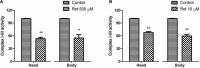
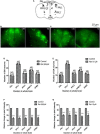
Parkinson's disease (PD) affects almost 1% of the population worldwide over the age of 50 years. Exposure to environmental toxins like paraquat and rotenone is a risk factor for sporadic PD which constitutes 95% of total cases. Herbicide rotenone has been shown to cause Parkinsonian symptoms in multiple animal models. Drosophila is an excellent model organism for studying neurodegenerative diseases (NDD) including PD. The aging process is characterized by differential expression of genes during different life stages. Hence it is necessary to develop life-stage-matched animal models for late-onset human disease(s) such as PD. Such animal models are critical for understanding the pathophysiology of age-related disease progression and important to understand if a genotropic drug/nutraceutical can be effective during late stages. With this idea, we developed an adult life stage-specific (health and transition phase, during which late-onset NDDs such as PD sets in) rotenone-mediated Drosophila model of idiopathic PD. Drosophila is susceptible to rotenone in dose-time dependent manner. Rotenone-mediated fly model of sporadic PD exhibits mobility defects (independent of mortality), inhibited mitochondrial complex I activity, dopaminergic (DAergic) neuronal dysfunction (no loss of DAergic neuronal number; however, reduction in rate-limiting enzyme tyrosine hydroxylase (TH) synthesis), and alteration in levels of dopamine (DA) and its metabolites; 3,4-Dihydroxyphenylacetic acid (DOPAC) and Homovanilic acid (HVA) in brain-specific fashion. These PD-linked behaviors and brain-specific phenotypes denote the robustness of the present fly model of PD. This novel model will be of great help to decipher life stage-specific genetic targets of small molecule mediated DAergic neuroprotection; understanding of which is critical for formulating therapeutic strategies for PD.
Keywords: Drosophila; Parkinson’s disease; dopamine; health phase; rotenone; transition phase.
Copyright © 2022 Ayajuddin, Phom, Koza, Modi, Das, Chaurasia, Thepa, Jamir, Neikha and Yenisetti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
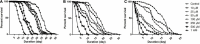

References
-
- Andersen A. D., Blaabjerg M., Binzer M., Kamal A., Thagesen H., Kjaer T. W., et al. (2017). Cerebrospinal fluid levels of catecholamines and its metabolites in Parkinson’s disease: Effect of l-DOPA treatment and changes in levodopa-induced dyskinesia. J. Neurochem. 141 614–625. 10.1111/jnc.13997 - DOI - PubMed
-
- Arking R. (2009). “Overview of the genomic architecture of longevity,” in Life span extension: Single cell organisms to man, eds Sell C., Lorenzini A., Brown-Borg H. M. (Dordrecht: Humana Press; ), 59–73. 10.1186/s12864-019-6066-6 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

