Advances in cell death mechanisms involved in viral myocarditis
- PMID: 36017100
- PMCID: PMC9395613
- DOI: 10.3389/fcvm.2022.968752
Advances in cell death mechanisms involved in viral myocarditis
Abstract
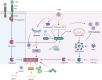
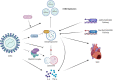
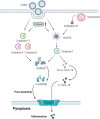
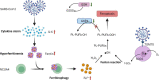
Viral myocarditis is an acute inflammatory disease of the myocardium. Although many etiopathogenic factors exist, coxsackievirus B3 is a the leading cause of viral myocarditis. Abnormal cardiomyocyte death is the underlying problem for most cardiovascular diseases and fatalities. Various types of cell death occur and are regulated to varying degrees. In this review, we discuss the different cell death mechanisms in viral myocarditis and the potential interactions between them. We also explore the role and mechanism of cardiomyocyte death with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Exploring the mechanisms may help in the early identification and the development of effective treatments, thus improving the quality of life of patients with viral myocarditis. We believe that the inhibition of cardiomyocyte death has immense therapeutic potential in increasing the longevity and health of the heart.
Keywords: CVB3; SARS-CoV-2; VMC; apoptosis; autophagy; ferroptosis; necrosis; pyroptosis.
Copyright © 2022 Yang, Li, You and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Lv S, Rong J, Ren S, Wu M, Li M, Zhu Y, et al. Epidemiology and diagnosis of viral myocarditis. Hellenic J Cardiol. (2013) 54:382–91. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

