Key ferroptosis-related genes in abdominal aortic aneurysm formation and rupture as determined by combining bioinformatics techniques
- PMID: 36017103
- PMCID: PMC9395677
- DOI: 10.3389/fcvm.2022.875434
Key ferroptosis-related genes in abdominal aortic aneurysm formation and rupture as determined by combining bioinformatics techniques
Abstract
Objectives: Abdominal aortic aneurysm (AAA) is a cardiovascular disease with high mortality and pathogenesis closely related to various cell death types, e.g., autophagy, apoptosis and pyroptosis. However, the association between AAA and ferroptosis is unknown.
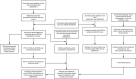
Methods: GSE57691 and GSE98278 dataset were obtained from the Gene Expression Omnibus database, and a ferroptosis-related gene (FRG) set was downloaded from the FerrDb database. These data were normalized, and ferroptosis-related differentially expressed genes (FDEGs, AAA vs. normal samples) were identified using the limma package in R. FRGs expression was analyzed by Gene Set Expression Analysis (GSEA), and FDEGs were analyzed by Gene Ontology (GO) and Kyoto Encyclopedia of Genes (KEGG) pathway enrichment analyses using the clusterProfiler package in R and ClueGO in Cytoscape. Protein-protein interaction networks were assembled using Cytoscape, and crucial FDEGs were identified using CytoHubba. Critical FDEG transcription factors (TFs) were predicted with iRegulon. FDEGs were verified in GSE98278 set, and key FDEGs in AAA (compared with normal samples) and ruptured AAA (RAAA; compared with AAA samples) were identified. Ferroptosis-related immune cell infiltration and correlations with key genes were analyzed by CIBERSORT. Key FEDGs were reverified in Ang II-induced AAA models of ApoE-/- and CD57B/6J mice by immunofluorescence assay.
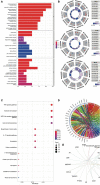
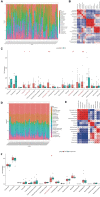
Results: In AAA and normal samples, 40 FDEGs were identified, and the expression of suppressive FRGs was significantly downregulated with GSEA. For FDEGs, the GO terms were response to oxidative stress and cellular response to external stimulus, and the KEGG pathways were the TNF and NOD-like receptor signaling pathways. IL6, ALB, CAV1, PTGS2, NOX4, PRDX6, GPX4, HSPA5, HSPB1, and NCF2 were the most enriched genes in the crucial gene cluster. CEBPG, NFAT5, SOX10, GTF2IRD1, STAT1, and RELA were potential TFs affecting these crucial genes. Ferroptosis-related immune cells involved in AAA formation were CD8+ T, naive CD4+ T, and regulatory T cells (Tregs); M0 and M2 macrophages; and eosinophils. Tregs were also involved in RAAA. GPX4, SLC2A1, and PEBP1 expression was downregulated in both the RAAA and AAA samples. GPX4 and PEBP1 were more important in AAA because they influenced ferroptosis-related immune cell infiltration, and SLC2A1 was more important in RAAA.
Conclusions: This is the first study to show that ferroptosis is crucial to AAA/RAAA formation. The TNF and NOD-like signaling pathways and ferroptosis-related immune cell infiltration play key roles in AAA/RAAA. GPX4 is a key ferroptosis-related gene in AAA. Ferroptosis and related genes might be promising targets in the treatment of AAA/RAAA.
Keywords: AAA (abdominal aortic aneurysm); GPX4; RAAA; ferroptosis; infiltration of immune cells.
Copyright © 2022 Ren, Lv, Wu, Chen, Lei, Yang, Li, Liu and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
[Exploration of key ferroptosis-related genes as therapeutic targets for sepsis based on bioinformatics and the depiction of their immune profiles characterization].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024 Oct;36(10):1025-1032. doi: 10.3760/cma.j.cn121430-20240524-00457. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024. PMID: 39586719 Chinese.
-
Exploration of key ferroptosis-related genes and immune infiltration in Crohn's disease using bioinformatics.Sci Rep. 2023 Aug 7;13(1):12769. doi: 10.1038/s41598-023-40093-w. Sci Rep. 2023. PMID: 37550393 Free PMC article.
-
Construction of the miRNA/Pyroptosis-Related Molecular Regulatory Axis in Abdominal Aortic Aneurysm: Evidence From Transcriptome Data Combined With Multiple Machine Learning Approaches Followed by Experiment Validation.J Immunol Res. 2024 Oct 30;2024:1429510. doi: 10.1155/2024/1429510. eCollection 2024. J Immunol Res. 2024. PMID: 39512836 Free PMC article.
-
Identification of hub ferroptosis-related genes and immune infiltration in lupus nephritis using bioinformatics.Sci Rep. 2022 Nov 5;12(1):18826. doi: 10.1038/s41598-022-23730-8. Sci Rep. 2022. PMID: 36335193 Free PMC article.
-
Patterns of immune infiltration in stable and raptured abdominal aortic aneurysms: A gene-expression-based retrospective study.Gene. 2020 Dec 15;762:145056. doi: 10.1016/j.gene.2020.145056. Epub 2020 Aug 15. Gene. 2020. PMID: 32805313
Cited by
-
Comprehensive Bioinformatics Analysis Reveals the Role of Shared Cuproptosis- and Ferroptosis-Related DEG DLD in Abdominal Aortic Aneurysm.J Cell Mol Med. 2025 Feb;29(3):e70399. doi: 10.1111/jcmm.70399. J Cell Mol Med. 2025. PMID: 39912406 Free PMC article.
-
Mesenchymal stem cell-derived extracellular vesicles protect against abdominal aortic aneurysm formation by inhibiting NET-induced ferroptosis.Exp Mol Med. 2023 May;55(5):939-951. doi: 10.1038/s12276-023-00986-2. Epub 2023 May 1. Exp Mol Med. 2023. PMID: 37121969 Free PMC article.
-
Ferroptosis: a new strategy for cardiovascular disease.Front Cardiovasc Med. 2023 Sep 4;10:1241282. doi: 10.3389/fcvm.2023.1241282. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37731525 Free PMC article. Review.
-
Inhibition of VSMC Ferroptosis Mitigates Pathological Vascular Remodeling: A Novel Therapeutic Strategy for Abdominal Aortic Aneurysm.J Cardiovasc Transl Res. 2025 Apr 21. doi: 10.1007/s12265-025-10621-2. Online ahead of print. J Cardiovasc Transl Res. 2025. PMID: 40259193
-
ALAS2 overexpression alleviates oxidative stress-induced ferroptosis in aortic aneurysms via GATA1 activation.J Thorac Dis. 2024 Apr 30;16(4):2510-2527. doi: 10.21037/jtd-24-370. Epub 2024 Apr 29. J Thorac Dis. 2024. PMID: 38738239 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

