Porous Silicon Nanoparticles Targeted to the Extracellular Matrix for Therapeutic Protein Delivery in Traumatic Brain Injury
- PMID: 36017941
- PMCID: PMC9492643
- DOI: 10.1021/acs.bioconjchem.2c00305
Porous Silicon Nanoparticles Targeted to the Extracellular Matrix for Therapeutic Protein Delivery in Traumatic Brain Injury
Abstract
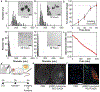
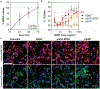
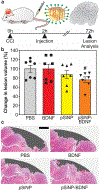
Traumatic brain injury (TBI) is a major cause of disability and death among children and young adults in the United States, yet there are currently no treatments that improve the long-term brain health of patients. One promising therapeutic for TBI is brain-derived neurotrophic factor (BDNF), a protein that promotes neurogenesis and neuron survival. However, outstanding challenges to the systemic delivery of BDNF are its instability in blood, poor transport into the brain, and short half-life in circulation and brain tissue. Here, BDNF is encapsulated into an engineered, biodegradable porous silicon nanoparticle (pSiNP) in order to deliver bioactive BDNF to injured brain tissue after TBI. The pSiNP carrier is modified with the targeting ligand CAQK, a peptide that binds to extracellular matrix components upregulated after TBI. The protein cargo retains bioactivity after release from the pSiNP carrier, and systemic administration of the CAQK-modified pSiNPs results in effective delivery of the protein cargo to injured brain regions in a mouse model of TBI. When administered after injury, the CAQK-targeted pSiNP delivery system for BDNF reduces lesion volumes compared to free BDNF, supporting the hypothesis that pSiNPs mediate therapeutic protein delivery after systemic administration to improve outcomes in TBI.
Conflict of interest statement
CONFLICT DISCLOSURE
MJS is a scientific founder (SF), member of the Board of Directors (BOD), Advisory Board (AB), Scientific Advisory Board (SAB), acts as a paid consultant (PC) or has an equity interest (EI) in the following: Aivocode, Inc (AB, EI); Bejing ITEC Technologies (SAB, PC); Cend Therapeutics (SF, BOD, EI); Illumina (EI); Matrix Technologies (EI); NanoVision Bio (SAB, EI); Pacific Integrated Energy (AB, EI); Quanterix (EI); Spinnaker Biosciences, Inc. (SF, BOD, EI); TruTag Technologies (SAB, EI); and Well-Healthcare Technologies (SAB, PC). MJS is also a Guest Professor at Zhejiang University, China. Although one or more of the grants that supported this research has been identified for conflict of interest management based on the overall scope of the project and its potential benefit to the companies listed, the research findings included in this publication may not necessarily relate to their interests. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
Figures