Protein palmitoylation in cancer: molecular functions and therapeutic potential
- PMID: 36018061
- PMCID: PMC9812842
- DOI: 10.1002/1878-0261.13308
Protein palmitoylation in cancer: molecular functions and therapeutic potential
Abstract
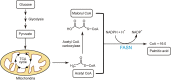
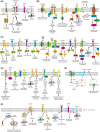
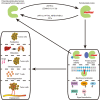
Protein S-palmitoylation (hereinafter referred to as protein palmitoylation) is a reversible lipid posttranslational modification catalyzed by the zinc finger DHHC-type containing (ZDHHC) protein family. The reverse reaction, depalmitoylation, is catalyzed by palmitoyl-protein thioesterases (PPTs), including acyl-protein thioesterases (APT1/2), palmitoyl protein thioesterases (PPT1/2), or alpha/beta hydrolase domain-containing protein 17A/B/C (ABHD17A/B/C). Proteins encoded by several oncogenes and tumor suppressors are modified by palmitoylation, which enhances the hydrophobicity of specific protein subdomains, and can confer changes in protein stability, membrane localization, protein-protein interaction, and signal transduction. The importance for protein palmitoylation in tumorigenesis has just started to be elucidated in the past decade; palmitoylation appears to affect key aspects of cancer, including cancer cell proliferation and survival, cell invasion and metastasis, and antitumor immunity. Here we review the current literature on protein palmitoylation in the various cancer types, and discuss the potential of targeting of palmitoylation enzymes or palmitoylated proteins for tumor treatment.
Keywords: cancer treatment; oncogene; protein S-palmitoylation; tumor suppressor; tumorigenesis.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. - PubMed
-
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. - PubMed
-
- Kim YC, Lee SE, Kim SK, Jang HD, Hwang I, Jin S, et al. Toll‐like receptor mediated inflammation requires FASN‐dependent MYD88 palmitoylation. Nat Chem Biol. 2019;15:907–16. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

