Neuroprotective role of Noggin in spinal cord injury
- PMID: 36018152
- PMCID: PMC9727439
- DOI: 10.4103/1673-5374.350190
Neuroprotective role of Noggin in spinal cord injury
Abstract
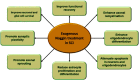
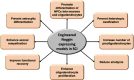
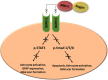
Spinal cord injury is one of the leading causes of morbidity and mortality among young adults in many countries including the United States. Difficulty in the regeneration of neurons is one of the main obstacles that leave spinal cord injury patients with permanent paralysis in most instances. Recent research has found that preventing acute and subacute secondary cellular damages to the neurons and supporting glial cells can help slow the progression of spinal cord injury pathogenesis, in part by reactivating endogenous regenerative proteins including Noggin that are normally present during spinal cord development. Noggin is a complex protein and natural inhibitor of the multifunctional bone morphogenetic proteins, and its expression is high during spinal cord development and after induction of spinal cord injury. In this review article, we first discuss the change in expression of Noggin during pathogenesis in spinal cord injury. Second, we discuss the current research knowledge about the neuroprotective role of Noggin in preclinical models of spinal cord injury. Lastly, we explain the gap in the knowledge for the use of Noggin in the treatment of spinal cord injury. The results from extensive in vitro and in vivo research have revealed that the therapeutic efficacy of Noggin treatment remains debatable due to its neuroprotective effects observed only in early phases of spinal cord injury but little to no effect on altering pathogenesis and functional recovery observed in the chronic phase of spinal cord injury. Furthermore, clinical information regarding the role of Noggin in the alleviation of progression of pathogenesis, its therapeutic efficacy, bioavailability, and safety in human spinal cord injury is still lacking and therefore needs further investigation.
Keywords: Noggin; apoptosis; astrocyte differentiation; axon myelination; axon regeneration; bone morphogenetic protein; glial scar; heterotrophic ossification; neurogenesis; neuropathic pain; spinal cord injury.
Conflict of interest statement
None
Figures
References
-
- Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury:a systematic review and meta-analysis. Eur J Pain. 2017;21:29–44. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

