Chronic spinal cord compression associated with intervertebral disc degeneration in SPARC-null mice
- PMID: 36018188
- PMCID: PMC9727435
- DOI: 10.4103/1673-5374.350210
Chronic spinal cord compression associated with intervertebral disc degeneration in SPARC-null mice
Abstract
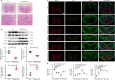
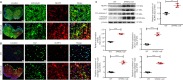
Chronic spinal cord compression (CSCC) is induced by disc herniation and other reasons, leading to movement and sensation dysfunction, with a serious impact on quality of life. Spontaneous disc herniation rarely occurs in rodents, and therefore establishing a chronic spinal cord compression (CSCC) animal model is of crucial importance to explore the pathogenesis and treatment of CSCC. The absence of secreted protein, acidic, and rich in cysteine (SPARC) leads to spontaneous intervertebral disc degeneration in mice, which resembles human disc degeneration. In this study, we evaluated whether SPARC-null mice may serve as an animal model for CSCC. We performed rod rotation test, pain threshold test, gait analysis, and Basso Mouse Scale score. Our results showed that the motor function of SPARC-null mice was weakened, and magnetic resonance images revealed compression at different spinal cord levels, particularly in the lumbar segments. Immunofluorescence staining and western blot assay showed that the absence of SPARC induced apoptosis of neurons and oligodendrocytes, activation of microglia/macrophages with M1/M2 phenotype and astrocytes with A1/A2 phenotype; it also activated the expression of the NOD-like receptor protein 3 inflammasome and inhibited brain-derived neurotrophic factor/tyrosine kinase B signaling pathway. Notably, these findings are characteristics of CSCC. Therefore, we propose that SPARC-null mice may be an animal model for studying CSCC caused by disc herniation.
Keywords: acidic; and rich in cysteine; apoptosis; astrocytes; chronic spinal cord compression; disc degeneration; disc herniation; macrophages; microglia; neuroinflammation; neurons; NOD-like receptor protein 3 inflammasomes; secreted protein.
Conflict of interest statement
None
Figures







Similar articles
-
Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model.Spine J. 2015 Dec 1;15(12):2524-37. doi: 10.1016/j.spinee.2015.08.055. Epub 2015 Aug 31. Spine J. 2015. PMID: 26334234
-
ISSLS Prize winner: Increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration.Spine (Phila Pa 1976). 2014 Aug 1;39(17):1345-54. doi: 10.1097/BRS.0000000000000334. Spine (Phila Pa 1976). 2014. PMID: 24718079
-
Low back pain and disc degeneration are decreased following chronic toll-like receptor 4 inhibition in a mouse model.Osteoarthritis Cartilage. 2018 Sep;26(9):1236-1246. doi: 10.1016/j.joca.2018.06.002. Epub 2018 Jun 18. Osteoarthritis Cartilage. 2018. PMID: 29908959
-
Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen.Ageing Res Rev. 2020 Jan;57:100978. doi: 10.1016/j.arr.2019.100978. Epub 2019 Oct 24. Ageing Res Rev. 2020. PMID: 31669486 Review.
-
Systematic Review of Outcomes Following 10-Year Mark of Spine Patient Outcomes Research Trial for Intervertebral Disc Herniation.Spine (Phila Pa 1976). 2020 Jun 15;45(12):825-831. doi: 10.1097/BRS.0000000000003400. Spine (Phila Pa 1976). 2020. PMID: 32004232
Cited by
-
Unveiling the role of TCF19 in intervertebral disc degeneration with single-cell and bulk RNA sequencing.Sci Rep. 2025 May 8;15(1):16043. doi: 10.1038/s41598-025-01180-2. Sci Rep. 2025. PMID: 40341079 Free PMC article.
-
TLR3 Knockdown Attenuates Pressure-Induced Neuronal Damage In Vitro.J Cell Mol Med. 2024 Dec;28(23):e70276. doi: 10.1111/jcmm.70276. J Cell Mol Med. 2024. PMID: 39671271 Free PMC article.
-
SPARC: a potential target for functional nanomaterials and drugs.Front Mol Biosci. 2023 Jul 28;10:1235428. doi: 10.3389/fmolb.2023.1235428. eCollection 2023. Front Mol Biosci. 2023. PMID: 37577749 Free PMC article. Review.
-
Functional Outcomes of Therapeutic Selective Nerve Root Block for Single-Segment Lumbar Spinal Stenosis: A Retrospective Study.Med Sci Monit. 2024 Jun 21;30:e943634. doi: 10.12659/MSM.943634. Med Sci Monit. 2024. PMID: 38905165 Free PMC article.
-
Prediction and validation of anoikis-related genes in neuropathic pain using machine learning.PLoS One. 2025 Feb 27;20(2):e0314773. doi: 10.1371/journal.pone.0314773. eCollection 2025. PLoS One. 2025. PMID: 40014587 Free PMC article.
References
-
- Adams BL, Guo W, Gors RT, Knopp KL. Pharmacological interrogation of a rodent forced ambulation model:leveraging gait impairment as a measure of pain behavior pre-clinically. Osteoarthritis Cartilage. 2016;24:1928–1939. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

