A hyaluronic acid granular hydrogel nerve guidance conduit promotes regeneration and functional recovery of injured sciatic nerves in rats
- PMID: 36018191
- PMCID: PMC9727441
- DOI: 10.4103/1673-5374.350212
A hyaluronic acid granular hydrogel nerve guidance conduit promotes regeneration and functional recovery of injured sciatic nerves in rats
Abstract
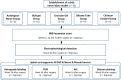
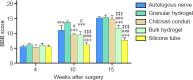
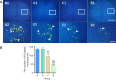
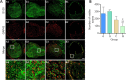
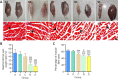
A hyaluronic acid granular hydrogel can promote neuronal and astrocyte colony formation and axonal extension in vitro, suggesting that the hydrogel can simulate an extracellular matrix structure to promote neural regeneration. However, in vivo experiments have not been conducted. In this study, we transplanted a hyaluronic acid granular hydrogel nerve guidance conduit to repair a 10-mm long sciatic nerve gap. The Basso, Beattie, and Bresnahan locomotor rating scale, sciatic nerve compound muscle action potential recording, Fluoro-Gold retrograde tracing, growth related protein 43/S100 immunofluorescence staining, transmission electron microscopy, gastrocnemius muscle dry/wet weight ratio, and Masson's trichrome staining results showed that the nerve guidance conduit exhibited similar regeneration of sciatic nerve axons and myelin sheath, and recovery of the electrophysiological function and motor function as autologous nerve transplantation. The conduit results were superior to those of a bulk hydrogel or silicone tube transplant. These findings suggest that tissue-engineered nerve conduits containing hyaluronic acid granular hydrogels effectively promote the morphological and functional recovery of the injured sciatic nerve. The nerve conduits have the potential as a material for repairing peripheral nerve defects.
Keywords: functional recovery; granular hydrogel; hyaluronic acid; myelin sheath; nerve conduit; nerve regeneration; peripheral nerve regeneration; sciatic nerve injury; tissue engineering; transection injury.
Conflict of interest statement
None
Figures
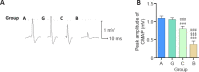


References
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Biazar E. Design of porous alginate conduit using a freeze drying technique for neural engineering. Orient J Chem. 2011;27:1029–1032.
LinkOut - more resources
Full Text Sources
Miscellaneous

