Genetic Modification of Primary Human Myeloid Cells to Study Cell Migration, Activation, and Organelle Dynamics
- PMID: 36018279
- PMCID: PMC9476234
- DOI: 10.1002/cpz1.514
Genetic Modification of Primary Human Myeloid Cells to Study Cell Migration, Activation, and Organelle Dynamics
Abstract
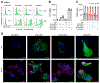
Myeloid dendritic cells (DCs) and macrophages are mononuclear phagocytes with key roles in the immune system. As antigen-presenting cells, they link innate detection of microbes with programming adaptive immune responses. Myeloid DCs and macrophages also play critical roles in development, promote tissue homeostasis, and direct repair in response to injury and inflammation. As cellular migration and organelle dynamics are intimately connected with these processes, it is necessary to develop tools to track myeloid cell behavior and function. Here, we build on previously established protocols to isolate primary human myeloid cells from peripheral blood and report an optimized method for their genetic modification with lentiviral vectors to study processes related to cell migration, activation, and organelle dynamics. Specifically, we provide a protocol for delivering genetically encoded fluorescent markers into primary monocyte-derived DCs (MDDCs) and monocyte-derived macrophages (MDMs) to label mitochondria, peroxisomes, and whole cells. We describe the isolation of primary CD14+ monocytes from peripheral blood using positive selection with magnetic beads and, alternatively, isolation based on plastic adherence. Isolated CD14+ cells can be transduced with lentiviral vectors and subsequently cultured in the presence of cytokines to derive MDDCs or MDMs. This protocol is highly adaptable for cotransduction with vectors to knock down or overexpress genes of interest. These tools enable mechanistic studies of genetically modified myeloid cells through flow cytometry, fluorescence microscopy, and other downstream assays. © 2022 Wiley Periodicals LLC. Basic Protocol: Transduction of MDDCs and MDMs with lentiviral vectors encoding fluorescent markers Alternate Protocol 1: Isolation of monocytes by plastic adhesion Alternate Protocol 2: Transduction of MDDCs and MDMs with lentiviral vectors to knock down or overexpress genes of interest Support Protocol 1: Production and purification of lentiviral vectors for transduction into primary human myeloid cells Support Protocol 2: Flow cytometry of MDDCs and MDMs Support Protocol 3: Fixed and live-cell imaging of fluorescent markers in MDMs and MDDCs.
Keywords: gene delivery; innate immunity; viral vectors.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures
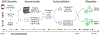

References
-
- Akkina RK, Walton RM, Chen ML, Li QX, Planelles V, & Chen IS (1996). High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol, 70(4), 2581–2585. doi:10.1128/JVI.70.4.2581-2585.1996 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

