Suprachoroidal Space Triamcinolone Acetonide: A Review in Uveitic Macular Edema
- PMID: 36018461
- PMCID: PMC9512860
- DOI: 10.1007/s40265-022-01763-7
Suprachoroidal Space Triamcinolone Acetonide: A Review in Uveitic Macular Edema
Erratum in
-
Correction to: Suprachoroidal Space Triamcinolone Acetonide: A Review in Uveitic Macular Edema.Drugs. 2022 Sep;82(13):1411. doi: 10.1007/s40265-022-01777-1. Drugs. 2022. PMID: 36074323 Free PMC article. No abstract available.
Abstract
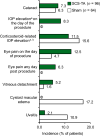
Triamcinolone acetonide injectable suspension for suprachoroidal use (Xipere®; SCS triamcinolone acetonide) is a corticosteroid approved in the USA for the treatment of macular edema associated with uveitis. Suprachoroidal injection of SCS triamcinolone acetonide results in preferential distribution into the posterior segment, which may reduce the risk of corticosteroid-related adverse events, such as cataracts and intraocular pressure (IOP) elevation. In a multicenter phase III trial in patients with non-infectious uveitic macular edema, SCS triamcinolone acetonide significantly and rapidly improved visual acuity and reduced signs of macular edema compared with sham treatment. SCS triamcinolone acetonide was generally well tolerated, with the most common adverse event being eye pain on the day of the procedure. The risk of corticosteroid-related IOP elevation appeared to be reduced in unrescued patients in the SCS triamcinolone acetonide group compared with patients in the sham control group who received rescue therapy. SCS triamcinolone acetonide is a novel and useful treatment option for uveitic macular edema.
Plain language summary
Uveitic macular edema is a major cause of blindness in the developed world. Intravitreal and periocular application of corticosteroids (e.g., triamcinolone acetonide) may be effective for uveitis and macular edema, but these routes are often associated with cataracts and corticosteroid-related intraocular pressure (IOP) elevation. Recently, a triamcinolone acetonide suspension for injection into the suprachoroidal space (Xipere®; SCS triamcinolone acetonide) has been approved for the treatment of uveitic macular edema. The suprachoroidal route preferentially distributes the drug to the back of the eye, resulting in a reduced risk of corticosteroid-related adverse events. In a pivotal clinical trial, SCS triamcinolone acetonide rapidly improved visual acuity and resolved macular edema in patients with non-infectious uveitis. SCS triamcinolone acetonide was generally well tolerated, with the most common ocular adverse event being eye pain on the day of procedure. In unrescued patients in the SCS triamcinolone acetonide group, there appeared to be a reduced risk of corticosteroid-related IOP elevation versus patients who received rescue therapy in the sham control group. SCS triamcinolone acetonide is a novel and useful treatment option for uveitic macular edema.
© 2022. Springer Nature.
Conflict of interest statement
Simon Fung and Yahiya Syed are salaried employees of Adis International Ltd/Springer Nature, and declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Figures
Similar articles
-
Treatment of Noninfectious Uveitic Macular Edema with Periocular and Intraocular Corticosteroid Therapies: A Report by the American Academy of Ophthalmology.Ophthalmology. 2024 Sep;131(9):1107-1120. doi: 10.1016/j.ophtha.2024.02.019. Epub 2024 Apr 20. Ophthalmology. 2024. PMID: 38647511 Review.
-
Periocular Triamcinolone vs. Intravitreal Triamcinolone vs. Intravitreal Dexamethasone Implant for the Treatment of Uveitic Macular Edema: The PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) Trial.Ophthalmology. 2019 Feb;126(2):283-295. doi: 10.1016/j.ophtha.2018.08.021. Epub 2018 Sep 27. Ophthalmology. 2019. PMID: 30269924 Free PMC article. Clinical Trial.
-
Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema.Retina. 2011 Jan;31(1):111-8. doi: 10.1097/IAE.0b013e3181e378af. Retina. 2011. PMID: 20856170
-
Suprachoroidal triamcinolone acetonide versus rescue therapies for the treatment of uveitic macular oedema: A post hoc analysis of PEACHTREE.Clin Exp Ophthalmol. 2022 Jan;50(1):23-30. doi: 10.1111/ceo.14024. Epub 2021 Dec 27. Clin Exp Ophthalmol. 2022. PMID: 34741564 Free PMC article. Clinical Trial.
-
Risk of intraocular pressure elevation associated with triamcinolone acetonide administration via different routes in macular edema: a systematic review and network meta-analysis of randomized controlled trials.BMC Ophthalmol. 2025 Mar 25;25(1):150. doi: 10.1186/s12886-025-03979-z. BMC Ophthalmol. 2025. PMID: 40128688 Free PMC article.
Cited by
-
Effect of suprachoroidal triamcinolone on intraocular pressure: a systematic review and meta-analysis.Ther Adv Ophthalmol. 2024 Feb 5;16:25158414241228671. doi: 10.1177/25158414241228671. eCollection 2024 Jan-Dec. Ther Adv Ophthalmol. 2024. PMID: 38327802 Free PMC article.
-
IOP Reduction in Nonhuman Primates by Microneedle Injection of Drug-Free Hydrogel to Expand the Suprachoroidal Space.Transl Vis Sci Technol. 2024 Oct 1;13(10):14. doi: 10.1167/tvst.13.10.14. Transl Vis Sci Technol. 2024. PMID: 39377753 Free PMC article.
-
A Case of Incidental and Uncomplicated Subretinal Triamcinolone Acetonide.Case Rep Ophthalmol. 2024 Jun 7;15(1):472-477. doi: 10.1159/000539190. eCollection 2024 Jan-Dec. Case Rep Ophthalmol. 2024. PMID: 39015233 Free PMC article.
-
Glucocorticoid-Induced Ocular Hypertension and Glaucoma.Clin Ophthalmol. 2024 Feb 16;18:481-505. doi: 10.2147/OPTH.S442749. eCollection 2024. Clin Ophthalmol. 2024. PMID: 38379915 Free PMC article. Review.
-
Use of corticosteroids in non-infectious uveitis - expert consensus in Taiwan.Ann Med. 2024 Dec;56(1):2352019. doi: 10.1080/07853890.2024.2352019. Epub 2024 May 15. Ann Med. 2024. PMID: 38747459 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

