Sensitivity of the Dorsal-Central Retinal Pigment Epithelium to Sodium Iodate-Induced Damage Is Associated With Overlying M-Cone Photoreceptors in Mice
- PMID: 36018572
- PMCID: PMC9428360
- DOI: 10.1167/iovs.63.9.29
Sensitivity of the Dorsal-Central Retinal Pigment Epithelium to Sodium Iodate-Induced Damage Is Associated With Overlying M-Cone Photoreceptors in Mice
Abstract
Purpose: Retinal pigment epithelium (RPE) degeneration is a leading cause of blindness in retinal degenerative diseases, but the mechanism of RPE regional degeneration remains largely unknown. This study aims to investigate the sensitivity of RPE to sodium iodate (SI) injury in the dorsal and ventral visual fields in mice and analyze whether overlaying cone photoreceptors regulate the sensitivity of RPE to SI-induced damage.
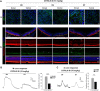
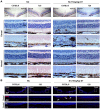
Methods: SI was used to induce RPE degeneration in mice. Hematoxylin-eosin staining, immunostaining, and TUNEL assay were used to evaluate retinal degeneration along the dorsal-ventral axis. Flat-mounted and sectional retinal immunostaining were used to analyze the distribution of cones along the dorsoventral axis in C57BL/6, albino, and 129 mice. Electroretinography was used to examine the retinal function.
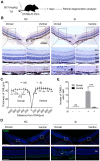
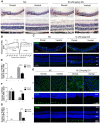
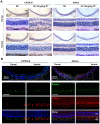
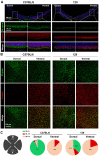
Results: Dorsal-central RPE was more sensitive to SI-mediated injury along the dorsal-ventral axis in C57BL/6 mice. Compared with the ventral RPE, the dorsal-central RPE was dominantly covered by M cone photoreceptors in these mice. Interestingly, M cone photoreceptor degeneration was followed by dorsal RPE degeneration under a low dose of SI. Furthermore, the sensitivity of dorsal RPE to a low dose of SI was reduced in both albino and 129 mouse strains with dominant mixed cones instead of M cones in the dorsal visual field.
Conclusions: These findings suggest that dorsal-central RPE is more sensitive to SI injury and that SI-induced RPE degeneration could be controlled by modifying the dominant overlying cone population in the mouse dorsal retina, thereby highlighting a potential role of M cones in RPE regional degeneration.
Conflict of interest statement
Disclosure:
Figures



References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev . 2005; 85: 845–881. - PubMed
-
- Ma X, Li H, Chen Y, et al. .. The transcription factor MITF in RPE function and dysfunction. Prog Retin Eye Res . 2019; 73: 100766. - PubMed
-
- Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci . 2014; 15: 615–627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

