Acetic Acid Enables Precise Tailoring of the Mechanical Behavior of Protein-Based Hydrogels
- PMID: 36018622
- PMCID: PMC9479135
- DOI: 10.1021/acs.nanolett.2c01558
Acetic Acid Enables Precise Tailoring of the Mechanical Behavior of Protein-Based Hydrogels
Abstract
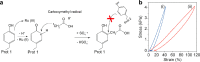
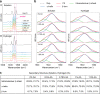
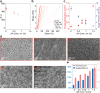
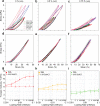
Engineering viscoelastic and biocompatible materials with tailored mechanical and microstructure properties capable of mimicking the biological stiffness (<17 kPa) or serving as bioimplants will bring protein-based hydrogels to the forefront in the biomaterials field. Here, we introduce a method that uses different concentrations of acetic acid (AA) to control the covalent tyrosine-tyrosine cross-linking interactions at the nanoscale level during protein-based hydrogel synthesis and manipulates their mechanical and microstructure properties without affecting protein concentration and (un)folding nanomechanics. We demonstrated this approach by adding AA as a precursor to the preparation buffer of a photoactivated protein-based hydrogel mixture. This strategy allowed us to synthesize hydrogels made from bovine serum albumin (BSA) and eight repeats protein L structure, with a fine-tailored wide range of stiffness (2-35 kPa). Together with protein engineering technologies, this method will open new routes in developing and investigating tunable protein-based hydrogels and extend their application toward new horizons.
Keywords: Biomaterials; Dynamic hydrogels; Protein folding transitions; Protein-based hydrogels; Responsive biomaterials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Chemical unfolding of protein domains induces shape change in programmed protein hydrogels.Nat Commun. 2019 Nov 29;10(1):5439. doi: 10.1038/s41467-019-13312-0. Nat Commun. 2019. PMID: 31784506 Free PMC article.
-
Photo-cross-linking approach to engineering small tyrosine-containing peptide hydrogels with enhanced mechanical stability.Langmuir. 2013 Oct 29;29(43):13299-306. doi: 10.1021/la4029639. Epub 2013 Oct 18. Langmuir. 2013. PMID: 24090141
-
Synthesis and characterization of photocrosslinkable albumin-based hydrogels for biomedical applications.Soft Matter. 2020 Oct 21;16(40):9242-9252. doi: 10.1039/d0sm00977f. Soft Matter. 2020. PMID: 32929420
-
Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications.Acta Biomater. 2017 Oct 15;62:42-63. doi: 10.1016/j.actbio.2017.07.028. Epub 2017 Jul 20. Acta Biomater. 2017. PMID: 28736220 Review.
-
Protein Hydrogels: The Swiss Army Knife for Enhanced Mechanical and Bioactive Properties of Biomaterials.Nanomaterials (Basel). 2021 Jun 24;11(7):1656. doi: 10.3390/nano11071656. Nanomaterials (Basel). 2021. PMID: 34202469 Free PMC article. Review.
Cited by
-
Green Hydrogel Synthesis: Emphasis on Proteomics and Polymer Particle-Protein Interaction.Polymers (Basel). 2022 Nov 6;14(21):4755. doi: 10.3390/polym14214755. Polymers (Basel). 2022. PMID: 36365747 Free PMC article. Review.
-
Design and Functionality of Trypsin-Triggered, Expandable Bovine Serum Albumin-Polyethylene Glycol Diacrylate Hydrogel Actuators.Small Sci. 2024 Jul 21;4(10):2400214. doi: 10.1002/smsc.202400214. eCollection 2024 Oct. Small Sci. 2024. PMID: 40212243 Free PMC article.
-
Toward Tunable Protein-Driven Hydrogel Lens.Adv Sci (Weinh). 2023 Dec;10(36):e2306862. doi: 10.1002/advs.202306862. Epub 2023 Nov 22. Adv Sci (Weinh). 2023. PMID: 37991134 Free PMC article.
-
Bovine Serum Albumin-Based Sponges as Biocompatible Adsorbents: Development, Characterization, and Perfluorooctane Sulfonate Removal Efficiency.Small Sci. 2025 Mar 2;5(4):2400497. doi: 10.1002/smsc.202400497. eCollection 2025 Apr. Small Sci. 2025. PMID: 40657189 Free PMC article.
References
-
- Gonzalez-Pujana A.; Vining K. H.; Zhang D. K. Y.; Santos-Vizcaino E.; Igartua M.; Hernandez R. M.; Mooney D. J. Multifunctional Biomimetic Hydrogel Systems to Boost the Immunomodulatory Potential of Mesenchymal Stromal Cells. Biomaterials 2020, 257, 120266.10.1016/j.biomaterials.2020.120266. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

