2'-O-Methylation of the second transcribed nucleotide within the mRNA 5' cap impacts the protein production level in a cell-specific manner and contributes to RNA immune evasion
- PMID: 36018811
- PMCID: PMC9458431
- DOI: 10.1093/nar/gkac722
2'-O-Methylation of the second transcribed nucleotide within the mRNA 5' cap impacts the protein production level in a cell-specific manner and contributes to RNA immune evasion
Abstract
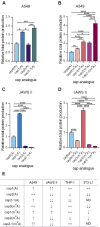
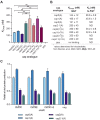
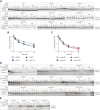
In mammals, m7G-adjacent nucleotides undergo extensive modifications. Ribose of the first or first and second transcribed nucleotides can be subjected to 2'-O-methylation to form cap1 or cap2, respectively. When the first transcribed nucleotide is 2'-O-methylated adenosine, it can be additionally modified to N6,2'-O-dimethyladenosine (m6Am). Recently, the crucial role of cap1 in distinguishing between 'self' and 'non-self' in mammalian cells during viral infection was revealed. Here, we attempted to understand the impact of cap methylations on RNA-related processes. Therefore, we synthesized tetranucleotide cap analogues and used them for RNA capping during in vitro transcription. Using this tool, we found that 2'-O-methylation of the second transcribed nucleotide within the mRNA 5' cap influences protein production levels in a cell-specific manner. This modification can strongly hamper protein biosynthesis or have no influence on protein production levels, depending on the cell line. Interestingly, 2'-O-methylation of the second transcribed nucleotide and the presence of m6Am as the first transcribed nucleotide serve as determinants that define transcripts as 'self' and contribute to transcript escape from the host innate immune response. Additionally, cap methylation status does not influence transcript affinity towards translation initiation factor eIF4E or in vitro susceptibility to decapping by DCP2; however, we observe the resistance of cap2-RNA to DXO (decapping exoribonuclease)-mediated decapping and degradation.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Akichika S., Hirano S., Shichino Y., Suzuki T., Nishimasu H., Ishitani R., Sugita A., Hirose Y., Iwasaki S., Nureki O.. Cap-specific terminal N6-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science. 2019; 363:eaav0080. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

