Soft-robotic ciliated epidermis for reconfigurable coordinated fluid manipulation
- PMID: 36026449
- PMCID: PMC9417179
- DOI: 10.1126/sciadv.abq2345
Soft-robotic ciliated epidermis for reconfigurable coordinated fluid manipulation
Abstract
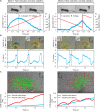
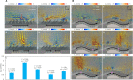
The fluid manipulation capabilities of current artificial cilia are severely handicapped by the inability to reconfigure near-surface flow on various static or dynamically deforming three-dimensional (3D) substrates. To overcome this challenge, we propose an electrically driven soft-robotic ciliated epidermis with multiple independently controlled polypyrrole bending actuators. The beating kinematics and the coordination of multiple actuators can be dynamically reconfigured to control the strength and direction of fluid transportation. We achieve fluid transportation along and perpendicular to the beating directions of the actuator arrays, and toward or away from the substrate. The ciliated epidermises are bendable and stretchable and can be deployed on various static or dynamically deforming 3D surfaces. They enable previously difficult to obtain fluid manipulation functionalities, such as transporting fluid in tubular structures or enhancing fluid transportation near dynamically bending and expanding surfaces.
Figures




References
-
- Gilpin W., Prakash V. N., Prakash M., Vortex arrays and ciliary tangles underlie the feeding-swimming trade-off in starfish larvae. Nat. Phys. 13, 380–386 (2017).
-
- Tamm S. L., Cilia and the life of ctenophores. Invertebr. Biol. 133, 1–46 (2014).
Grants and funding
LinkOut - more resources
Full Text Sources

