S100A8/A9 drives the formation of procoagulant platelets through GPIbα
- PMID: 36026606
- PMCID: PMC10653093
- DOI: 10.1182/blood.2021014966
S100A8/A9 drives the formation of procoagulant platelets through GPIbα
Abstract
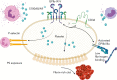
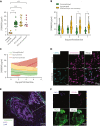
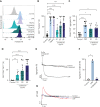
S100A8/A9, also known as "calprotectin" or "MRP8/14," is an alarmin primarily secreted by activated myeloid cells with antimicrobial, proinflammatory, and prothrombotic properties. Increased plasma levels of S100A8/A9 in thrombo-inflammatory diseases are associated with thrombotic complications. We assessed the presence of S100A8/A9 in the plasma and lung autopsies from patients with COVID-19 and investigated the molecular mechanism by which S100A8/A9 affects platelet function and thrombosis. S100A8/A9 plasma levels were increased in patients with COVID-19 and sustained high levels during hospitalization correlated with poor outcomes. Heterodimeric S100A8/A9 was mainly detected in neutrophils and deposited on the vessel wall in COVID-19 lung autopsies. Immobilization of S100A8/A9 with collagen accelerated the formation of a fibrin-rich network after perfusion of recalcified blood at venous shear. In vitro, platelets adhered and partially spread on S100A8/A9, leading to the formation of distinct populations of either P-selectin or phosphatidylserine (PS)-positive platelets. By using washed platelets, soluble S100A8/A9 induced PS exposure but failed to induce platelet aggregation, despite GPIIb/IIIa activation and alpha-granule secretion. We identified GPIbα as the receptor for S100A8/A9 on platelets inducing the formation of procoagulant platelets with a supporting role for CD36. The effect of S100A8/A9 on platelets was abolished by recombinant GPIbα ectodomain, platelets from a patient with Bernard-Soulier syndrome with GPIb-IX-V deficiency, and platelets from mice deficient in the extracellular domain of GPIbα. We identified the S100A8/A9-GPIbα axis as a novel targetable prothrombotic pathway inducing procoagulant platelets and fibrin formation, in particular in diseases associated with high levels of S100A8/A9, such as COVID-19.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: This work is protected by UoB patent (Patent Application Number: PCT/GB2022/052007). The authors declare no competing financial interests.
Figures










References
-
- Russwurm S, Vickers J, Meier-Hellmann A, et al. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock. 2002;17(4):263–268. - PubMed
-
- Nicolai L, Massberg S. Platelets as key players in inflammation and infection. Curr Opin Hematol. 2020;27(1):34–40. - PubMed
-
- Zhou J, Xu E, Shao K, et al. Circulating platelet-neutrophil aggregates as risk factor for deep venous thrombosis. Clin Chem Lab Med. 2019;57(5):707–715. - PubMed
-
- Marquardt L, Anders C, Buggle F, et al. Leukocyte-platelet aggregates in acute and subacute ischemic stroke. Cerebrovasc Dis. 2009;28(3):276–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

