European society of clinical microbiology and infectious diseases guidelines for coronavirus disease 2019: an update on treatment of patients with mild/moderate disease
- PMID: 36028088
- PMCID: PMC9398787
- DOI: 10.1016/j.cmi.2022.08.013
European society of clinical microbiology and infectious diseases guidelines for coronavirus disease 2019: an update on treatment of patients with mild/moderate disease
Abstract
Scope: Despite the large availability of vaccines, coronavirus disease 2019 (COVID-19), induced by severe acute respiratory syndrome coronavirus 2, continues to be a major threat for health-care providers and fragile people. A number of options are now available for outpatients with mild-to-moderate COVID-19 at the risk of disease progression for the prevention of deaths or hospitalization.
Methods: A European Society of Clinical Microbiology and Infectious Diseases COVID-19 guidelines task force was established by the European Society of Clinical Microbiology and Infectious Diseases Executive Committee. A small group was established, half appointed by the chair and the remaining selected based on an open call. Each panel met virtually once a week. For all decisions, a simple majority vote was used. A long list of clinical questions using the population, intervention, comparison, outcome format was developed at the beginning of the process. For each population, intervention, comparison, outcome, two panel members performed a literature search, with a third panelist involved in case of inconsistent results. Voting was based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
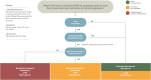
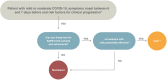
Recommendations: In this update, we focus on anti-viral agents, monoclonal antibodies (mAbs) and other treatment options proposed for patients with mild or moderate COVID-19 who are at the risk of hospitalization or death. Although the use of anti-virals is recommended, especially nirmatrelvir/ritonavir and remdesivir or, alternatively, molnupirarvir, the administration of mAbs against the spike protein strictly depends on circulating variants or the ability to test timely for variants and sub-variants. At the time of writing (April-June 2022), the only active mAb was tixagevimab/cilgavimab given the predominance of the Omicron BA.2, BA.3, BA.4 and BA.5 sub-lineages in Europe. However, considering that the epidemiological scenario is extremely dynamic, constant monitoring of variants of concern is mandatory.
Keywords: COVID-19; Cilgavimab; ESCMID; Molnupiravir; Nirmatrelvir/ritonavir; Outpatients; Remdesivir; Sotrovimab; Tixagevimab.
Copyright © 2022 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- World Health Organization . 2020. Clinical management of COVID-19.https://www.who.int/publications-detail/clinical-management-of-severe-ac...
-
- European Centre for Disease Prevention and Control (ECDC) 2021. Risk factors and risk groups.https://www.ecdc.europa.eu/en/covid-19/latest-evidence/risk-factors-risk...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

