Donepezil inhibits neuromuscular junctional acetylcholinesterase and enhances synaptic transmission and function in isolated skeletal muscle
- PMID: 36028305
- PMCID: PMC9826304
- DOI: 10.1111/bph.15940
Donepezil inhibits neuromuscular junctional acetylcholinesterase and enhances synaptic transmission and function in isolated skeletal muscle
Abstract
Background and purpose: Donepezil, a piperidine inhibitor of acetylcholinesterase (AChE) prescribed for treatment of Alzheimer's disease, has adverse neuromuscular effects in humans, including requirement for higher concentrations of non-depolarising neuromuscular blockers during surgery. Here, we examined the effects of donepezil on synaptic transmission at neuromuscular junctions (NMJs) in isolated nerve-muscle preparations from mice.
Experimental approach: We measured effects of therapeutic concentrations of donepezil (10 nM to 1 μM) on AChE enzymic activity, muscle force responses to repetitive stimulation, and spontaneous and evoked endplate potentials (EPPs) recorded intracellularly from flexor digitorum brevis muscles from CD01 or C57BlWldS mice.
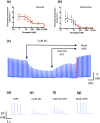
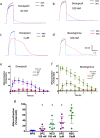
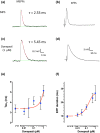
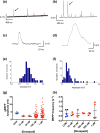
Key results: Donepezil inhibited muscle AChE with an approximate IC50 of 30 nM. Tetanic stimulation in sub-micromolar concentrations of donepezil prolonged post-tetanic muscle contractions. Preliminary Fluo4-imaging indicated an association of these contractions with an increase and slow decay of intracellular Ca2+ transients at motor endplates. Donepezil prolonged spontaneous miniature EPP (MEPP) decay time constants by about 65% and extended evoked EPP duration almost threefold. The mean frequency of spontaneous MEPPs was unaffected but the incidence of 'giant' MEPPs (gMEPPs), some exceeding 10 mV in amplitude, was increased. Neither mean MEPP amplitude (excluding gMEPPs), mean EPP amplitude, quantal content or synaptic depression during repetitive stimulation were significantly altered by concentrations of donepezil up to 1 μM.
Conclusion and implications: Adverse neuromuscular signs associated with donepezil therapy, including relative insensitivity to neuromuscular blockers, are probably due to inhibition of AChE at NMJs, prolonging the action of ACh on postsynaptic nicotinic acetylcholine receptors but without substantively impairing evoked ACh release.
Keywords: Alzheimer's disease; acetylcholine; acetylcholinesterase; anticholinesterase; endplate potential; muscle contraction; neuromuscular block; neuromuscular junction.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
None of the authors has any conflict of interests.
Figures
Similar articles
-
Role of acetylcholinesterase on the structure and function of cholinergic synapses: insights gained from studies on knockout mice.Cell Mol Neurobiol. 2011 Aug;31(6):909-20. doi: 10.1007/s10571-011-9690-5. Epub 2011 May 3. Cell Mol Neurobiol. 2011. PMID: 21538119 Free PMC article.
-
Increased quantal size in transmission at slow but not fast neuromuscular synapses of apolipoprotein E deficient mice.Exp Neurol. 2004 Feb;185(2):290-6. doi: 10.1016/j.expneurol.2003.10.006. Exp Neurol. 2004. PMID: 14736510
-
Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockout mouse neuromuscular junctions.Br J Pharmacol. 2003 Jan;138(1):177-87. doi: 10.1038/sj.bjp.0705010. Br J Pharmacol. 2003. PMID: 12522088 Free PMC article.
-
Giant miniature endplate potentials at vertebrate neuromuscular junctions: A review.Eur J Neurosci. 2024 Dec;60(12):7183-7194. doi: 10.1111/ejn.16624. Epub 2024 Nov 26. Eur J Neurosci. 2024. PMID: 39600045 Review.
-
Calcium-insensitive miniature endplate potentials at the neuromuscular junction.Synapse. 1987;1(4):281-92. doi: 10.1002/syn.890010402. Synapse. 1987. PMID: 2843994 Review.
Cited by
-
Effects of Donepezil on the Musculoskeletal System in Female Rats.Int J Mol Sci. 2023 May 19;24(10):8991. doi: 10.3390/ijms24108991. Int J Mol Sci. 2023. PMID: 37240337 Free PMC article.
References
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021). The concise guide to pharmacology 2021/22: Enzymes. British Journal of Pharmacology, 178(Suppl 1), S313–S411. 10.1111/bph.15542 - DOI - PubMed
-
- Alexander, S. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Buneman, O. P. , Cidlowski, J. A. , Christopoulos, A. , Davenport, A. P. , Fabbro, D. , Spedding, M. , Striessnig, J. , Davies, J. A. , Ahlers‐Dannen, K. E. , … Zolghadri, Y. (2021). The concise guide to pharmacology 2021/22: Introduction and other protein targets. British Journal of Pharmacology, 178 Suppl 1, S1–S26. 10.1111/bph.15537 - DOI - PMC - PubMed
-
- Alexander, S. P. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Aldrich, R. W. , Attali, B. , Baggetta, A. M. , Becirovic, E. , Biel, M. , Bill, R. M. , Catterall, W. A. , … Zhu, M. (2021). The concise guide to pharmacology 2021/22: Ion channels. British Journal of Pharmacology, 178(Suppl 1), S157–S245. 10.1111/bph.15539 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

