Asymmetric 1,4-functionalization of 1,3-enynes via dual photoredox and chromium catalysis
- PMID: 36028488
- PMCID: PMC9418150
- DOI: 10.1038/s41467-022-32614-4
Asymmetric 1,4-functionalization of 1,3-enynes via dual photoredox and chromium catalysis
Abstract
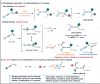
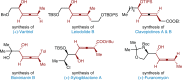
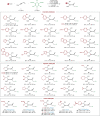
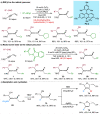
The merger of photoredox and transition-metal catalysis has evolved as a robust platform in organic synthesis over the past decade. The stereoselective 1,4-functionalization of 1,3-enynes, a prevalent synthon in synthetic chemistry, could afford valuable chiral allene derivatives. However, tremendous efforts have been focused on the ionic reaction pathway. The radical-involved asymmetric 1,4-functionalization of 1,3-enynes remains a prominent challenge. Herein, we describe the asymmetric three-component 1,4-dialkylation of 1,3-enynes via dual photoredox and chromium catalysis to provide chiral allenols. This method features readily available starting materials, broad substrate scope, good functional group compatibility, high regioselectivity, and simultaneous control of axial and central chiralities. Mechanistic studies suggest that this reaction proceeds through a radical-involved redox-neutral pathway.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Fu L, Greßies S, Chen P, Liu G. Recent advances and perspectives in transition metal‐catalyzed 1,4‐functionalizations of unactivated 1,3‐enynes for the synthesis of allenes. Chin. J. Chem. 2019;38:91–100. doi: 10.1002/cjoc.201900277. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

